Abstract
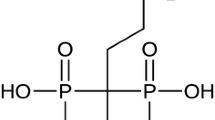
In order to reduce the pain due bone metastases a last generation bisphosphonate, 2-(3-pyridinyl)-1-hydroxyethane diphosphonic acid, was labeled with 188Re and investigated further. Labeling was performed at 95 °C for 15 min. The stability of radioconjugate was checked in human serum at 37 °C and biodistribution was studied in balb/c mice. Labeling yield of ≥98 % was obtained corresponding to a specific activity of 46 MBq/µmol. The radioconjugate showed good stability in human serum. Our main achievement was high bone uptake (4.24 ± 0.12 % ID/g at 24 h post injection) which may therapeutically be beneficial for the palliation of painful bone metastasis.





Similar content being viewed by others
References
Yoneda T, Sasaki A, Mundy GR (1994) Osteolytic bone metastasis in breast cancer. Breast Cancer Res Treat 32:73–84
Mercadante S (1997) Malignant bone pain: pathophysiology and treatment. Pain 69:1–18
Uppelschoten JM, Wanders SL, de Jong JM (1995) Single-dose radiotherapy (6 Gy): palliation in painful bone metastases. Radiother Oncol 36:198–202
Di Lorenzo G, Autorino R, Ciardiello F, Raben D, Bianco C, Troiani T (2003) Bone metastases are a severe problem in oncology, since they usually are associated with pain. Oncol Rep 10:399–404
McEwan AJ (2000) Use of radionuclides for the palliation of bone metastases. Semin Radiat Oncol 10:103–114
Pandit-Taskar N, Batraki M, Divgi CR (2004) Radiopharmaceutical therapy for palliation of bone pain from osseous metastases. J Nucl Med 45:1358–1365
Elder RC, Yuan J, Helmer B, Pipes D, Deutsch K, Deutsch E (1997) Studies of the structure and composition of rhenium-1,1-hydroxyethylidenediphosphonate (HEDP) analogues of the radiotherapeutic agent (186)ReHEDP. Inorg Chem 36:3055–3063
Lam MG, de Klerk JM, van Rijk PP (2004) 186Re-HEDP for metastatic bone pain in breast cancer patients. Eur J Nucl Med Mol Imaging 31(Suppl 1):S162–S170
Castronovo EP, Callahan RJ (1972) New bone scanning agent: 99mTc-labeled 1-hydroxy-ethylidene-1, 1-disodium phosphonate. J Nucl Med 13:823–827
Cocquyt V, Kline WF, Gertz BJ, van Belle SJ, Holland SD, DeSmet M, Quan H, Vyas KP, Zhang KE, De Greve J, Porras AG (1999) Pharmacokinetics of intravenous alendronate. J Clin Pharmacol 39:385–393
Bushinsky DA, Neumann KJ, Asplin J, Krieger NS (1999) Alendronate decreases urine calcium and supersaturation in genetic hypercalciuric rats. Kidney Int 55:234–243
Smith MR (2008) Osteoclast targeted therapy for prostate cancer: bisphosphonates and beyond. Urol Oncol: Semin Orig Investig 26:420–425
Neves M, Gano L, Pereira N, Costa MC, Costa MR, Chandia M, Rosado M, Fausto R (2002) Synthesis, characterization and biodistribution of bisphosphonates Sm-153 complexes: correlation with molecular modeling interaction studies. Nucl Med Biol 29:329–338
Reginster J, Minne HW, Sorensen OH, Hooper M, Roux C, Brandi ML, Lund B, Ethgen D, Pack S, Roumagnac I, Eastell R (2000) Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. Osteoporos Int 11(1):83–91
Maxon HR III, Schroder LE, Thomas SR, Hertzberg VS, Deutsch EA, Scher HI (1990) Re-186(Sn) HEDP for treatment of painful osseous metastases: initial clinical experience in 20 patients with hormone-resistant prostate cancer. Radiology 176:155–159
Robinson RJ (1990) Systemic radioisotopic therapy of primary and metastatic bone cancer. J Nucl Med 31:1326–1327
Palmedo H, Guhlke S, Bender H, Sartor J, Schoeneich G, Risse J, Grunwald F, Knapp FF Jr, Biersack HJ (2000) Dose escalation study with rhenium-186 HEDP in prostate cancer patients with osseous metastases. Eur J Nucl Med 27:123–130
Arano Y, Ono M, Wakisaka K, Uezono T, Akizawa H, Motonari Y (1995) Synthesis and biodistribution studies of 186Re complex of 1-hydroxyethylidene-1,1-diphosphonate for treatment of painful osseous metasitases. Radioisotopes 44:514–522
De Winter F, Brans B, Van De Wiele C, Dierckx RA (1999) Visualization of the stomach on rhenium-186 HEDP imaging after therapy for metastasized prostate carcinoma. Clin Nucl Med 24:898–899
Limouris GS, Skukla SK (1997) Gastric uptake in Re-186 HEDP bone scintigraphy. Anticancer Res 17:1779–1782
Deutsch E, Libson K, Vanderheyden JL, Ketring AR, Maxon HR (1986) The chemistry of rhenium and technetium as related to the use of isotopes of these elements in therapeutic and diagnostic nuclear medicine. Nucl Med Biol 4:465–477
Verdera ES, Gaudiano J, Leon A, MartõÂnez G, Robles A, Savio E (1997) Rhenium-188-HEDP kit formulation and quality control. Radiochim Acta 79:113–117
Bai HS, Jin XH, Wang F, Du J, Liu YM, Chen DM (1996) Preparation of a cold kit of186Re(Sn)-HEDP. J Radioanal Nucl Chem 206:43–50
Janoki GA, Polyak A, Kiraly R, Balogh L, Korosi L, Mathe D (2007) Comparative evaluation of therapeutic radiopharmaceuticals. International atomic energy agency, Vienna, p 103
Lin WY, Hsieh JF, Lin CP, Hsieh BT, Ting G, Wang SJ, Knapp FFR (1999) Effect of reaction conditions on preparations of rhenium-188 hydroxyethylidene diphosphonate complexes. Nucl Med Biol 26:455–459
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Erfani, M., Doroudi, A., Dinari, M.A. et al. Preparation of a rhenium-188 labeled bisphosphonate for bone pain palliation therapy. J Radioanal Nucl Chem 303, 2027–2032 (2015). https://doi.org/10.1007/s10967-014-3813-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-014-3813-7