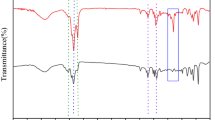
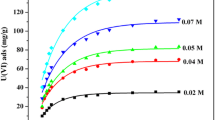
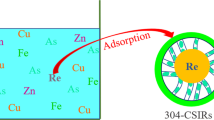
Abstract
Mutual separation of Th(IV) and U(VI) ions is very important in industry. Two newly solvent impregnated resins (SIRs) were prepared for this investigation. One of the SIRs contents 1,4-diaminoantraquinone (DAAQ) and the other one contains 1,4-dihydroxyantraquinone and DAAQ as 1:1 mixed-ligands. Comparison of adsorption behavior of Th(IV) and U(VI) on both types the SIRs were carried out using several models. The results showed that the adsorption behavior of the mixed-ligands SIR is methodically differs from the other one so that the successful separation of U(VI) from Th(IV) is feasible using the mixed-ligands SIR.








Similar content being viewed by others
Abbreviations
- A T :
-
Temkin constant (mg L−1)
- b :
-
Langmuir constant related to the free energy of adsorption (L mg−1)
- B T :
-
Temkin constant (L g−1)
- C 0 :
-
The initial metal ion concentration (mg L−1)
- C e :
-
The equilibrium concentration of metal ion in the balk solution (mg L−1)
- I :
-
The intercept of intraparticle diffusion eq. related to boundary layer thickness
- K F :
-
Frendlich constant indicative of the relative adsorption capacity of SIR (mg1−(1/n) L1/n g−1)
- k 1 :
-
Rate constant of pseudo-first-order adsorption (min−1)
- k 2 :
-
Rate constant of pseudo-second-order adsorption (g mg−1 min)
- k i :
-
Rate constant of the intraparticle diffusion (mg g−1 min−0.5)
- n :
-
Frendlich constant indicative of the intensity of the adsorption
- q e :
-
The amount of metal ion adsorbed per unit weight of SIR at equilibrium (mg g−1)
- q m :
-
The theoretical monolayer saturation capacity (mg g−1)
- q t :
-
The amount of metal ion adsorbed per unit weight of SIR at time t (mg g−1)
- R L :
-
Separation factor, also called equilibrium parameter
- T :
-
Temperature (K)
- t :
-
Contact time (min)
- α :
-
Elovich equation, the initial adsorption rate (mg g−1 min)
- β :
-
Elovich equation, the parameter related to the extent of surface coverage and activation energy for chemisorption b (g mg−1)
References
Clayton GD, Clayton FE (eds), (1994) Patty’s Industrial Hygiene and Toxicology. 2C 2735
Agency for Toxic Substances and Disease Registry (2000) U.S. Public Health Service, Chapman and Hall, New York
Condamines N, Musikas C (1992) The extraction by N.N-dialkylamides. II. Extraction of actinide cations. Solvent Extr Ion Exch 10:69–100
Mowafy EA, Aly HF (2002) Extraction behaviours of Nd(III), Eu(III), La(III), Am(III), and U(VI) with some substituted malonamides from nitrate medium. Solvent Extr Ion Exch 20:177–194
Ardois C, Musikas C, Fattahi M, Abbe AC (1992) Selective actinide solvent extraction used in conjunction with liquid scintillation. J Radioanal Nucl Chem 226:241–245
Gupta B, Malik P, Deep A (2002) Extraction of uranium, thorium and lanthanides using Cyanex-923: Their separations and recovery from monazite. J Radioanal Nucl Chem 251:451–456
Maheswari MA, Subramanian MS (2004) Selective enrichment of U(VI), Th(IV) and La(III) from high acidic streams using a new chelating ion-exchange polymeric matrix. Talanta 64:202–209
Prabhakaran D, Subramanian MS (2005) Selective extraction of U(VI) over Th(IV) from acidic streams using di-bis(2-ethylhexyl) malonamide anchored chloromethylated polymeric matrix. Talanta 65:179–184
Prabhakaran D, Subramanian MS (2004) Selective extraction of U(VI), Th(IV), and La(III) from acidic matrix solutions and environmental samples using chemically modified Amberlite XAD-16 resin. Anal Bioanal Chem 379:519–525
Jain VK, Pandya RA, Pillai SG, Shrivastav PS (2006) Simultaneous preconcentration of uranium(VI) and thorium(IV) from aqueous solutions using a chelating calix[4]arene anchored chloromethylated polystyrene solid phase. Talanta 70:257–266
Kabay N, Gizli N, Demirel N, Demircioglu M, Yuksel M, Saglam M (2003) Removal of cadmium from phosphoric acid solutionby solvent-impregnated resins (sirs) sorption kinetics and equilibria studies. Chem Eng Comm 190:936–947
Karve M, Rajgor RV (2008) Amberlite XAD-2 impregnated organophosphinic acid extractant for separation of uranium(VI) from rare earth elements. Desalination 232:191–197
Singh BN, Maiti B (2006) Separation and preconcentration of U(VI) on XAD-4 modified with 8-hydroxy quinolone. Talanta 69:303–396
Merdivan M, Pirinccioglu N, Hamamci C (2003) Thorium(IV) and uranium(VI) adsorption studies on octacarboxymethyl-C-methylcalix[4] resorcinarene impregnated on a polymeric support. Anal Chim Acta 485:213–219
Metwally E, Saleh ASh, El-Naggar HA (2005) Extraction and Separation of Uranium (VI) and Thorium (IV) Using Tri-n-dodecylamine Impregnated Resins. J Radioanal Nucl Chem 6:119–126
Hosseini MS, Hosseini-Bandegharaei A (2010) Selective extraction of Th(IV) over U(VI) and other co-existing ions using eosin B-impregnated Amberlite IRA-410 resin beads. J Radioanal Nucl Chem 283:23–30
Hosseini-Bandegharaei A, Hosseini MS, Jalalabadi Y et al (2013) A novel extractant-impregnated resin containing carminic acid for selective separation and pre-concentration of uranium(VI) and thorium(IV). Int J Environ Anal Chem 93:108–124
Hosseini MS, Abedi F (2014) Stepwise extraction of Th(IV) and U(VI) ions with mixed-ligands impregnated resin containing 1,4-diaminoanthraquinone and 1,4-dihydroxyanthraquinone. J Radioanal Nucl Chem. doi:10.1007/s10967-014-3366-9
Hosseini MS, Hosseini M, Hosseini-Bandegharaei A (2007) Solvent impregnated resins containing quinizarin: preparation and application to batch-mode separation of Cd(II), Cu(II), Ni(II), and Zn(II) in aqueous media prior to the determination by flame atomic absorption spectrometry. Sep Sci Technol 42:3465–3480
Hosseini MS, Hosseini-Bandegharaei A, Hosseini M (2009) Column-mode separation and pre-concentration of some heavy metal ions by solvent-impregnated resins containing quinizarin before the determination by flame atomic absorption spectrometry. Int J Environ Anal Chem 89:35–48
Hosseini MS, Hosseini-Bandegharaei A (2011) Comparison of adsorption behavior of Th(IV) and U(VI) on modified impregnated resin containing quinizarin with that conventional prepared impregnated resin. J Hazard Mater 190:755–765
Aveyard R, Haydon DA (1973) An Introduction to the Principles of Surface Chemistry. Cambridge University Press, Cambridge, p 201
Langmuir I (1916) The constitution and fundamental properties of solids and liquids. J Am Chem Soc 38:2221–2295
Freundlich H (1906) Adsorption in solution. Phys Chem Soc 40:1361–1368
Ozacar M, Sengil IA (2005) Adsorption of metal complex dyes from aqueous solutions by pine sawdust. Bioresour Technol 96:791–795
Hall KR, Eagleton LC, Acrivos A, Vermeulen T (1966) Pore and solid diffusion kinetics in fixed-bed adsorption under constant pattern conditions. Ind Eng Chem Fundam 5:212–223
Bulut E, Ozacar M, Sengil IA (2008) Equilibrium and kinetic data and process design for adsorption of Congo red onto bentonite. J Hazard Mater 154:613–622
Mane VS, Mall ID, Srivastava VC (2007) Use of bagasse fly ash as an adsorbent for the removal of brilliant green dye from aqueous solution. Dyes Pigments 73:269–278
McKay G, Ho YS (1999) Pseudo-second-order model for adsorption processes. Process Biochem 34:451–465
Chein SH, Clayton WR (1980) Application of Elovich equation to the biomass of phosphate release and adsorption on soil. Soil Sci Soc Am J 44:265–268
Weber WJ, Morris JC, Sanit J (1963) Kinetics of adsorption on carbon from solution. Eng Div Am Soc Civil Eng 89:31–60
Ho Y-S (2004) Citiation review of Lagergren kinetic rate equation on adsorption reactions. Scientometrics 59: 171–177
Acknowledgments
The authors wish to thank the University of Birjand and Islamic Azad University-Neyshabur, Iran for their financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hosseini, M.S., Abedi, F. Comparison of adsorption behavior of Th(IV) and U(VI) on mixed-ligands impregnated resin containing antraquinones with that conventional one. J Radioanal Nucl Chem 303, 2173–2183 (2015). https://doi.org/10.1007/s10967-014-3755-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-014-3755-0