Abstract
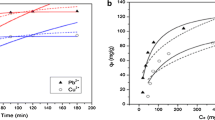
The interaction of Eu(III) with two different types of marine sediments has been investigated as a function of various parameters (e.g. pH, [Eu(III)]o, I, humic acid) to evaluate their effect on the Eu(III) adsorption. The adsorption properties (e.g. adsorption capacity) of the different sediment types, as well as the effect of the physicochemical parameters differ significantly from one type to another, depending on the (surface) composition. Generally, the adsorption data are well described by the Langmuir isotherm and the formation of outer-sphere complexes is favoured at low pH, whereas the formation of inner-sphere complexes becomes predominant at pH > 4.





Similar content being viewed by others
References
Holm E, Fukai R (1986) Actinide isotopes in the marine environment. J Less Common Met 122:487–497
Tsiaili A, Kiliari T, Pashalidis I (2011) Seasonal variation of the alpha-radioactivity concentration in natural water systems in Cyprus. Radiat Meas 46:145–148
Pathak PN, Choppin GR (2007) Sorption of Am 3+ cations on suspended silicate: effects of pH, ionic strength, complexing anions, humic acid and metal ions. J Radioanal Nucl Chem 274:517–523
Kim JI (1993) The chemical behavior of transuranium elements and barrier functions in natural aquifer systems. Proceedings from materials research society symposium, 1993
Choppin GR (2007) Actinide speciation in the environment. J Radioanal Nucl Chem 273:695–703
Seaborg GT (1993) Overview of the actinide and lanthanide (the f) elements. Radiochim Acta 61:115–122
Khalifa SM, El-Atrash AM, Helal AA, Aly HF (1989) Sorption of europium (III), cobalt (II) and cesium (I) by fresh water sediment fractions. Isotopenpraxis 25:335–340
Tang J, Johannesson KH (2010) Rare earth elements adsorption onto Carrizo sand: influence of strong solution complexation. Chem Geol 279:120–133
Tang J, Johannesson KH (2005) Adsorption of rare earth elements onto Carrizo sand: experimental investigations and modeling with surface complexation. Geochim Cosmochim Acta 69:5247–5261
Coppin F, Berger G, Bauer A, Castet S, Loubet M (2002) Sorption of lanthanides on smectite and kaolinite. Chem Geol 182:57–68
Kosmulski M (1997) Adsorption of trivalent cations on silica. J Colloid Interface Sci 195:395–403
Lippold H, Muller N, Kupsch H (2005) Effect of humic acid on the pH-dependent adsorption of terbium(III) onto geological materials. Appl Geochem 20:1209–1217
Tertre E, Hofmann A, Berger G (2008) Rare earth element sorption by basaltic rock: experimental data and modeling results using the “generalised composite approach”. Geochim Cosmochim Acta 72:1043–1056
Konstantinou M, Kolokassidou K, Pashalidis I (2009) Studies on the interaction of olive cake and its hydrophylic extracts with polyvalent metal ions (Cu(II), Eu(III)) in aqueous solutions. J Hazard Mater 166:1169–1173
Efstathiou M, Pashalidis I (2013) A comparative study of the adsorption of uranium on commercial and natural (Cypriot) sea sand samples. J Radioanal Nucl Chem 298:1111–1116
Lu YW, Laurent G, Pereira H (2004) A novel methodology for evaluation of formation constants of complexes: example of lanthanide-Arsenazo III complexes. Talanta 62:959–970
Nguyen C, Do DD (2001) The Dubinin–Radushkevich equation and the underlying microscopic adsorption description. Carbon 39:1327–1336
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liatsou, I., Efstathiou, M. & Pashalidis, I. Adsorption of trivalent lanthanides by marine sediments. J Radioanal Nucl Chem 304, 41–45 (2015). https://doi.org/10.1007/s10967-014-3448-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-014-3448-8