Abstract
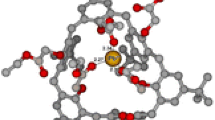
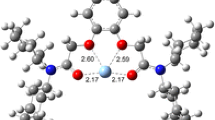
By using extraction experiments and γ-activity measurements, the extraction constant corresponding to the equilibrium Sr2+(aq) + 2A−(aq) + 1(nb) ⇔ 1·Sr2+(nb) + 2A−(nb) occurring in the two-phase water–nitrobenzene system (A− = picrate, 1 = antamanide; aq = aqueous phase, nb = nitrobenzene phase) was determined as log K ex (1·Sr2+, 2A−) = −0.3 ± 0.1. Further, the stability constant of the 1·Sr2+ complex in nitrobenzene saturated with water was calculated for a temperature of 25 °C: log β nb (1·Sr2+) = 8.8 ± 0.1. Finally, applying quantum mechanical density functional level of theory calculations, the most probable structure of the cationic complex species 1·Sr2+ was derived. In the resulting complex, the “central” cation Sr2+ is bound by six bond interactions to the corresponding six oxygen atoms of the parent ligand 1. The interaction energy of the considered 1·Sr2+ complex was found to be −1,114.9 kJ/mol, confirming the formation of this cationic species as well.




Similar content being viewed by others
References
Wieland T, Faulstich H, Burgemeister W (1972) Biochem Biophys Res Commun 47:984
Ruzza P, Calderan A, Biondi B, Carrara M, Tancredi T, Borin G (1999) J Pept Res 53:442
Wieland T, Lüben G, Ottenheym H, Faesel J, de Vries JX, Prox A, Schmid J (1968) Angew Chem Int Ed Engl 7:204
Wieland T, Faulstich H (1978) Crit Rev Biochem 5:185
Moran AM, Park SM, Mukamel S (2003) J Chem Phys 118:9971
Falvo C, Hayashi T, Zhuang W, Mukamel S (2008) J Phys Chem B 112:12479
Ruzza P, Calderan A, Carrara M, Tancredi T, Borin G (1997) Curr Topics Pept Protein Res 2:21
Rais J (1971) Collect Czech Chem Commun 36:3253
Makrlík E, Vaňura P (2010) J Radioanal Nucl Chem 285:683
Makrlík E, Hálová J, Kyrš M (1984) Collect Czech Chem Commun 49:39
Makrlík E, Vaňura P (1998) ACH Models Chem 135:213
Makrlík E, Toman P, Vaňura P (2013) J Mol Struct 1032:155
Lee C, Yang W, Parr RG (1988) Phys Rev B 37:785
Becke AD (1993) J Chem Phys 98:5648
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, Revision C.01. Gaussian, Inc, Wallingford, CT
Kříž J, Dybal J, Makrlík E, Budka J, Vaňura P (2009) J Phys Chem A 113:5896
Kříž J, Toman P, Makrlík E, Budka J, Shukla R, Rathore R (2010) J Phys Chem A 114:5327
Kříž J, Dybal J, Makrlík E, Vaňura P, Moyer BA (2011) J Phys Chem B 115:7578
Acknowledgments
This work was supported by the Grant Agency of Faculty of Environmental Sciences, Czech University of Life Sciences, Prague, Project No.: 42900/1312/3114 “Environmental Aspects of Sustainable Development of Society”, and by the Czech Ministry of Education, Youth, and Sports (Project MSM 6046137307).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Makrlík, E., Vaňura, P., Böhm, S. et al. Extraction and theoretical study on complexation of the strontium cation with antamanide. J Radioanal Nucl Chem 300, 1291–1294 (2014). https://doi.org/10.1007/s10967-014-3098-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-014-3098-x