Abstract
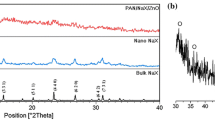
For the first time, effects of CuO nanoparticles concentration (from 1 to 24.2 wt%) in CuO/NaX nanocomposite and replacing various cations (Ag+, K+, Ca2+, and Mg2+) with Na+ ions in NaX zeolite on removal of uranium ions from drinking water are reported. The removal of uranium was performed under natural conditions of pH, laboratory temperature and the presence of competing cations and anions that are available in tap water of Isfahan city. Characterization of parent NaX zeolite and modified samples were investigated using X-ray fluorescence, X-ray powder diffraction patterns, scanning electron microscopy, and atomic absorption spectroscopy methods. Using Langmuir, Freundlich, and C-models, isotherms of equilibrium adsorption were studied. Results show the removal efficiency and distribution coefficient of NaX zeolite decrease in the presence of other competing anions and cations that exist in drinking water. But, modification of NaX zeolite with various cations and CuO nanoparticles might enhance the ability of X zeolite in removing uranium from drinking water.








Similar content being viewed by others
References
Stalder E, Blanc A, Haldimann M, Dudler V (2012) Chemosphere 86:672–679
Kumar S, Loganathan VA, Gupta RB, Barnett MO (2011) J Environ Manag 92:2504–2512
Birke M, Rauch U, Lorenz H, Kringel R (2010) J Geochem Explor 107:272–282
Nriagu J, Nam DH, Ayanwola TA, Dinh H, Erdenechimeg E, Ochir C, Bolorma TA (2012) Sci Total Environ 414:722–726
Carvalho FP, Oliveira JM (2010) Environ Int 36:352–360
Sidhu SH, Keith-Roach MJ, Lloyd JR, Vaughan DJ (2010) Sci Total Environ 408:5690–5700
Milja TE, Prathish KP, Rao TP (2011) J Hazard Mater 188:384–390
Fathizadeh M, Aroujalian A, Raisi A (2011) J Membr Sci 375:88–95
Aydin FA, Soylak M (2007) Talanta 72:187–192
Shinde NR, Bankar AV, Kumar AR, Zinjarde SS (2012) J Environ Manag 102:115–124
Agrawal YK, Shrivastav P, Menon SK (2000) Sep Purif Technol 200:177–183
Fan FL, Qin Z, Bai J, Rong WD, Fan FY, Tian W, Lei XW, Wang Y, Zhao L (2012) J Environ Radioact 106:40–46
Nilchi A, Shariati Dehaghan T, Rasouli Garmarodi S (2013) Desalination 321:67–71
Krestou A, Xenidis A, Panias D (2003) Miner Eng 16:1363–1370
Baybas D, Ulusoy U (2011) J Hazard Mater 187:241–249
Mar Camachoa L, Denga S, Parra RR (2010) J Hazard Mater 75:393–398
Weihua Z, Lei Z, Runping H (2009) Chin J Chem Eng 17(4):585–593
Kilincarslan Kaygun A, Akyil S (2007) J Hazard Mater 147:357–362
Olguin MT, Solache-Rios M, Acosta D, Bosch P, Bulbulian S (1999) Micropor Mesopor Mater 28:377–385
Akyil S, Aslani MAA, Aytas SO (1998) J Alloy Compd 271/273:769–773
Aboul-Fotouh SMK, Hassan MMI (2010) Acta Chim Slov 57:872–879
Sebastian J, Mohan Jinka K, Vir Jasra R (2006) J Catal 244:208–218
Schwartzberg AM, Zhang JZ (2008) J Phys Chem C 112:10323–10337
Zaidi HA, Pant KK (2005) Can J Chem Eng 83:970–977
Bouvy C, Marine W, Sporken R, Su BL (2007) Colloid Surf A 300:145–149
Huang M, Xu C, Wu Z, Huang Y, Lin J, Wu J (2008) Dyes Pigments 77:327–334
Shakur HR (2011) Physica E 44:641–646
Singh BK, Tomar R, Tomar R, Tomar SS (2011) Micropor Mesopor Mater 142:629–640
Hernández-Montoya V, Pérez-Cruz MA, Mendoza-Castillo DI, Moreno-Virgen MR, Bonilla-Petriciolet A (2013) J Environ Manag 116:213–221
Taffarel SR, Rubio J (2010) Miner Eng 23:1131–1138
Shaheen SM, Eissa FI, Ghanem KM, Gamal El-Din HM, Al Anany FS (2013) J Environ Manag 128:514–521
Kumar Jha V, Nagae M, Matsuda M, Miyake M (2009) J Environ Manag 90:2507–2514
Limousin G, Gaudet JP, Charlet L, Szenknect S, Barthes V, Krimissa M (2007) Appl Geochem 22:249–275
Reed BE, Matsumoto MR (1993) Sep Sci Technol 28:2179–2195
McKay G, Otterburn MS, Sweeney AG (1981) Water Res 14:14–20
Ozer A, Pirincci HB (2006) J Hazard Mater B137:849–855
Jie Y, Dinghua Y, Peng S, He H (2011) Chin J Catal 32(3):405–411
Zhang J, Singh R, Webley PA (2008) Micropor Mesopor Mater 111:478–487
Kovacheva P, Arishtirova K, Vassilev S (2001) Appl Catal A Gen 210:391–395
Huang Q, Xue X, Zhou R (2011) J Mol Catal A 344:74–82
Mahdavi S, Jalali M, Afkhami A (2012) J Nanopart Res 14:846–864
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abdi, M.R., Shakur, H.R., Rezaee Ebrahim Saraee, K. et al. Effective removal of uranium ions from drinking water using CuO/X zeolite based nanocomposites: effects of nano concentration and cation exchange. J Radioanal Nucl Chem 300, 1217–1225 (2014). https://doi.org/10.1007/s10967-014-3092-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-014-3092-3