Abstract
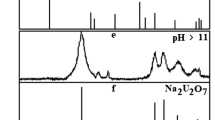
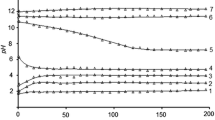
Uranyl silicates with formula MII(HSiUO6)2·6H2O (MII=Mn, Co, Ni, Cu, Zn) were investigated in aqueous solutions in the pH range of 0÷14. The pH interval was established where compounds preserve their composition and structure. It varies in the pH range of (3.5–4.0)÷(10.8–11.4) and depends on MII type. Out of this pH interval investigated uranyl silicates convert to the compounds of other composition and structure, such as amorphous silica, polyuranates and hydroxides of 3d-transition elements. The solubility of MII(HSiUO6)2·6H2O was determined, it’s value changes on the several orders of magnitude from 10−6 M in subalkali solutions to 10−3 M in acid and strongly alkaline media. Using obtained experimental data the solubility products and solubility curves of uranyl silicates were calculated by mathematical modeling. Also the speciation diagrams of uranium (VI), silicon (IV) and M (II) in solutions and solids were plotted.





Similar content being viewed by others
References
Lehmann S, Geipel G, Foerstendorf H, Bernhard G (2008) J Radioanal Nucl Chem 275:633–642
Vochten RA et al (1997) Can Mineral 35:735–741
Chernorukov NG, Knyazev AV, Nipruk OV (2003) Radiochemistry 49:300–304
Chernorukov NG, Knyazev AV, Sergacheva IV (2005) Russ J Inorg Chem 50:1–11
Vochten R, Blaton N, Peeters O (1997) Neues Jahrbuch fur Mineralogie Monatshefte 12:569–576
Rosenzweig A, Ryan RR (1975) Amer Mineral 60:448–453
Ryan RR, Rosenzweig A (1977) Cryst Struct Comm 6:611
Stohl FV, Smith DK (1981) Amer Mineral 66:610–625
Viswanathan K, Harneit O (1986) Amer Mineral 71:1489–1493
Ginderow D (1988) Acta Crystallogr A 44:421–424
Nguyen SN, Silva RJ, Weed HC, Andrews JE (1992) J Chem Thermodyn 24:359–376
Moll H, Geipel G, Matz W et al (1996) Radiochim Acta 74:3–7
Shvareva TY, Mazeina L, Gorman-Lewis D, Burns PC et al (2011) Geochim Cosmochim Acta 75:5269–5282
Ilton ES, Liu CX, Yantasee W et al (2006) Geochim Cosmochim Acta 70:4836–4849
Perez I, Casas I, Martin M, Bruno J (2000) Geochim Cosmochim Acta 64:603–608
Gorman-Lewis D, Burns PC, Fein JB (2008) J Chem Thermodyn 40:335–352
Miyshlyaeva LV, Krasnoschekova VV (1972) Analiticheskaya khimiya kremniya (Analytical chemistry of Silicon). Nauka, Moskow
Lazarev AI, Kharlamov IP, Yakovlev PYa et al (1976) Spravochnik khimika-analitika. Metallurgiya, Moskow
Vinogradov AP (1962) Analiticheskaya khimiya urana. Akademiya nauk, Moskow
Markov VK, Vernyi EA, Vinogradov AV et al (1964) Uran. Metody ego opredeleniya (Uranium. Determination Methods). Atomizdat, Moscow
Lavrukhina AK, Yukina LV (1974) Analiticheskaya khimiya margantsa (Analytical Chemistry of Manganese). Nauka, Moscow
Pyatnitskii IV (1965) Analiticheskaya khimiya kobal’ta (Analytical Chemistry of Cobalt). Nauka, Moscow
Peshkova VM, Savostina VM (1966) Analiticheskaya khimiya nikelya (Analytical Chemistry of Nickel). Nauka, Moscow
Podchainova VN, Simonova LN (1990) Analiticheskaya khimiya medi (Analytical Chemistry of Copper). Nauka, Moscow
Zhivopistsev VP, Selezneva EA (1975) Analiticheskaya khimiya tsinka (Analytical Chemistry of Zinc). Nauka, Moscow
Guillaumont R, Fanghanel T, Fuger J et al (2003) Update on the chemical thermodynamics of uranium, neptunium, and plutonium, 2nd edn. Elsevier, Amsterdam
Moll H, Geipel G, Brendler V, Bernhard G, Nitshe HJ (1998) Alloys Compd 271–273:765–768
Glushko VP (1965–1981)Termicheskie konstanty veshchestv (Thermal Constants of Substances). Akad. Nauk SSSR, Moscow
Martell AE (2001) NIST critically selected stablity constants of metal complexes, database, 6.0 for windows. US Department of Commerce, Technology Administration, National Institute of Standards and Technology, Gaithersburg, MD
Grenthe I, Fuger J, Koning R et al (2004) Chemical thermodynamics of uranium. North-Holland, Amsterdam
Kiseleva EK, Suslennikova VM (1959) Spravochnoe rukovodstvo po prigotovleniyu titrovannykh rastvorov I ustanovke ikh titrov (handbook on preparation of titrated solutions and determination of their titers). Akad. im. A.F, Mozhaiskogo
Chernorukov NG, Nipruk OV, Suleimanov EV, Pykhova JuP (2009) Radiochemistry 51:388–395
Jr Boisen et al (1994) Phys Chem Miner 21:269
Kovba LM (1972) Radiochemistry 14:727–730
Szytula A, Murasik A, Balanda M (1971) Phys Stat Sol 43:125–128
Database of Ionic Radii. http://abulafia.mt.ic.ac.uk/shannon/ptable.php
Acknowledgments
The investigation was finance supported by Federal Goal Program “Scientific and scientific-pedagogical cadres of innovative Russia 2009–2013” on direction “Radiochemistry. Chemistry of high energies” (No. 844P).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nipruk, O., Chernorukov, N., Zakharycheva, N. et al. State of uranyl silicates MII(HSiUO6)2 ·6H2O (MII=Mn, Co, Ni, Cu, Zn) in aqueous solutions. J Radioanal Nucl Chem 298, 519–529 (2013). https://doi.org/10.1007/s10967-013-2544-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-013-2544-5