Abstract
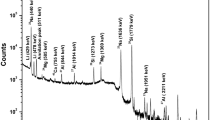
In this work k0-INAA (via IAEAk0-software) has been applied on glass samples to determine major, minor and trace element concentration. As many as 50 elements were detected and quantified with 3–5 mg of 0.1 % AuAl comparator monitor (0.1 % gold–99.9 %Alumimum wire). The average concentration of SiO2, Na2O, CaO, Al2O3 and MgO ranged between 76–96 %, 11.15–12.66 %, 5.26–10.71 %, 1.13–2.73 % and 3.51–6.23 % respectively. The relative concentrations of impurity elements; Cr, Fe, Mn and Co determined from the glass samples were used to match the physical appearance (color) of the glass based on general knowledge of colored glass production. The analytical procedure was validated using SRM 610 (glass matrix) and SRM GBW07106 (rock matrix) both as control samples which indicated a relative uncertainty of 15 and 6 % respectively for SRM 610 and SRM GBW07106. The relative sensitivity at which some of the elements were detected in major, minor and trace levels have indicated, that the k0-method in instrumental neutron activation analysis using low power research reactor is a useful technique in glass analysis and could equally be used for forensic and archeological glass characterization.
Similar content being viewed by others
References
Ojovan MI (2004) Glass formation in amorphous SiO2 as a percolation phase transition in a system of network defects”. JETP Lett 79(12):632–634
de Jong BHWS (1989) Glass. In: Elvers B, Hawkins S, Ravenscroft M, Rounsaville JF, Schultz G (eds) Ullmann’s encyclopedia of industrial chemistry, vol A12, 5th edn. VCH Publishers, Weinheim, Germany, pp 365–432
Pfaender HG (1996) Schott guide to glass. Springer, New York, pp 135, 186
Thomas PS, Terese V (2005) High temperature glass melt property database for process modeling. The American Ceramic Society, Westerville, OH. ISBN: 1-57498-225-7
David MI (2006) Substances used in the making of coloured glass. http://1st-glass.things.com/articles/glasscolouring.html. Retrieved 28 August 2012
Sayre EV (1963) The intentional use of antimony and manganese in ancient glasses. In: Mattson FR, Rindone GE (eds) Advances in glass technology Part 2. Plenum Press, New York, pp 263–282
Scientific Working Group for Material Analysis (SWGMAT) (2004) Elemental analysis of glass. (Google: pdf, retrieved August 2012)
Wedepohl KH (1997) Chemical composition of medieval glass from excavations in West Germany. Glastech Ber Glass Sci Technol 70:246–255
Wolf S, Kessler CM, Stern WB, Gerber Y (2005) The composition and manufacture of early medieval coloured window glass from Sion (Valois, Switzerland)—a Roman glass making tradition or innovative craftsmanship? Archaeometry 47:361–380
Sayre EV, Smith RW (1961) Compositional categories of ancient glass. Science 133:1824–1826
Hughes JC, Catterick T, Southeard G (1976) The quantitative analysis of glass by atomic absorption spectroscopy. Forensic Science 8:217–227
Hickman DA (1987) Glass types identified by chemical analysis. Forensic Sci Int 33:23–46
Andrasko J, Maehly AC (1978) The discrimination between samples of window glass by combining physical and chemical techniques. J Forensic Sci 23:250–262
Haney MA (1977) Comparison of window glasses by isotope dilution spark source mass spectrometry. J Forensic Sci 22:534–544
Almirall J (2001) Elemental analysis of glass fragments. In: Caddy B (ed) Trace evidence analysis and interpretation: glass and paint. Taylor and Francis, London, pp 65–83
Almirall JR, Trejos T (2006) Advances in the forensic analysis of Glass fragments with a focus on refractive index and elemental analysis. Forensic Sci Rev 18:2
Duckworth DC, Bayne CK, Morton SJ, Almirall JR (2000) Analysis of variance of forensic glass analysis by ICP-MS: variance within the method. J Anal At Spectrom 15:821
Koons RD, Peters CA, Rebbert PS (1991) Comparison of refractive index, energy dispersive X-ray fluorescence and inductively coupled plasma atomic emission spectrometry for forensic characterization of sheet glass fragments. J Anal At Spectrom 6:451
Parouchais T, Warner IM, Palmer LT, Kobus H (1996) The analysis of small glass fragments using inductively coupled plasma mass spectrometry. J Forensic Sci 41:351
Suzuki Y, Sugita R, Suzuki S, Marumo Y (2000) Forensic discrimination of bottle glass by refractive index measurement and analysis of trace element with ICP-MS. Anal Sci 16:1195
Duckworth DC, Morton SJ, Bayne K, Montero S, Koons RD, Almiral JR (2002) Forensic glass analysis by ICP-MS: a multi-element assessment of discriminating power via analysis of variance and pairwise comparisons. J Anal At Spectrom 17:662
Trejos T, Montero S, Almirall JR (2003) Analysis and comparison of glass fragments by laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS) and ICP-MS. Anal Bioanal Chem 376:1255
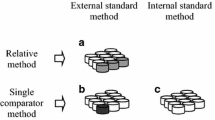
Girardi F, Guzzi G, Pauly J (1965) Reactor neutron activation analysis by the single comparator method. Anal Chem 37:1085–1092
De Corte F (1987) The k0 standardization method—a move to the optimization of NAA, thesis. Rijksuniversiteit Ghent, Ghent
Matthias R, Blaauw M (2006) Progress report in the k0-IAEA program, Elsevier. Nucl Instrum Methods Phys Res A 564:698–701
Simonits A, De Corte F, Hoste J (1975) Single comparator methods in reactor neutron activation analysis. J Radioanal Chem 24:31–46
Simonits A, De Corte F, Hoste J (1975) J Radioanal Nucl Chem 1:31
Baidoo IK (2012) Validation of IAEAk0-INAA software using a low power research reactor GHARR-1, Mphil, thesis. University of Ghana, Legon
Coleman RF, Goode GC (1973) Comparison of glass fragments by neutron activation analysis. J Radioanal Chem 15:367–388
Pitts SJ, KrotochiI B (1991) Statistical discrimination of flat glass fragments by instrumental neutron activation analysis method for forensic applications. J Forensic Sci JFSCA 36(1):122–137
Freestone IC, Ponting M, Hughes J (2002) The origins of Byzantine glass from Maroni Petrera, Cyprus. Archaeometry 44:257–272
Aerts A, Velde B, Janssens K, Dijkman W (2003) Change in silica sources in Roman and post-Roman glass. Spectrochim Acta Part B 58:659–667
Shortland AJ, Rogers N, Eremin K (2007) Trace element discriminants between Egyptian and Mesopotamian late Bronze Age glasses. J Archaeol Sci 34:781–789
Silvestri A, Molin G, Salviulo G (2008) The colourless glass of Iulia Felix. J Archaeol Sci 35:331–341
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Baidoo, I.K., Nyarko, B.J.B., Akaho, E.H.K. et al. Application of k0-method in instrumental neutron activation analysis to glass matrix: test study for low power research reactor GHARR-1. J Radioanal Nucl Chem 295, 1893–1901 (2013). https://doi.org/10.1007/s10967-012-2249-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-012-2249-1