Abstract
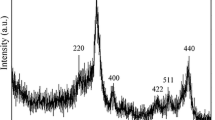
A novel γ-MnO2 hollow structure has been synthesized using a simple chemical reaction between MnSO4 and KMnO4 in aqueous solution without using any templates, surfactants, catalysts, calcination and hydrothermal processes. As an example of potential applications, γ-MnO2 hollow structure was used as adsorbent in radionuclide 60Co(II) treatment, and showed an excellent ability. The effect of pH, contact time, ionic strength, humic acid (HA)/fulvic acid (FA), and temperature was investigated using batch techniques. The results indicated that the sorption of 60Co(II) on γ-MnO2 was obviously dependent on pH values but independent of ionic strength. The presence of HA/FA enhanced the sorption of 60Co(II) on γ-MnO2 at low pH, whereas reduced 60Co(II) sorption on γ-MnO2 at high pH. The kinetic sorption of 60Co(II) on γ-MnO2 can be well fitted by the pseudo-second-order rate equation. The thermodynamic parameters (ΔH 0, ΔS 0, ΔG 0) were also calculated from the temperature dependent sorption isotherms, and the results suggested that the sorption of 60Co(II) on γ-MnO2 was a spontaneous and endothermic process. The sorption of 60Co(II) on γ-MnO2 was attributed to surface complexation rather than ion exchange.













Similar content being viewed by others
References
Yu SM, Li XG, Ren AP, Shao DD, Chen CL, Wang X (2006) J Radioanal Nucl Chem 268(2):387–392
Kudesia V P(1990) Water pollution. Pragati Prakashan, Meerut, pp 84–102
Rengaraj S, Moon SH (2002) Water Res 36:1783–1793
Yu SM, Ren AP, Chen CL, Chen YX, Wang XK (2006) Appl Radiat Isot 64:455–461
Chen CL, Xu D, Tan XL, Wang XK (2007) J Radioanal Nucl Chem 273:227–233
Yu SM, Ren AP, Cheng J, Song XP, Chen C, Wang X (2007) J Radioanal Nucl Chem 273:129–133
Chen L, Yu XJ, Zhao ZD, Pan JS (2008) J Radioanal Nucl Chem 275:209–216
Khan SA (2003) J Radioanal Nucl Chem 258:3–6
El-Khouly SH (2006) J Radioanal Nucl Chem 270:391–398
Lopez H, Olguin MT, Bosch P, Bulbulian S (1995) J Radioanal Nucl Chem 200:19–23
Khan SA, Rehman RU, Khan MA (1996) J Radioanal Nucl Chem 207:19–37
Shakir K, Flex H, Benyamin K (1993) J Radioanal Nucl Chem 173:303–311
Shahwan T, Erten HN (1999) J Radioanal Nucl Chem 241:151–155
Dong WM, Wang XK, Shen Y, Zhao XD, Tao ZY (2000) J Radioanal Nucl Chem 245:431–434
Todorov B, Pekov G, Djingova R (2008) J Radioanal Nucl Chem 278:9–15
Wua CH, Linb CF, Mab HW, Hsib TQ (2003) Water Res 37:743–752
Tan XL, Chang PP, Fan QH, Zhou X, Yu SM, WuWS WangXK (2008) Colloid Surf A 328:8–14
Benaissa H, Benguella B (2004) Environ Pollut 130:157–163
Lead JR, Hamilton-Taylor J, Peters A, Reiner S, Tipping E (1998) Anal Chim Acta 369:171–180
Kinniburgh DG, Milne CJ, Benedetti MF, Pinheiro JP, Filius J, Koopal LK (1996) Environ Sci Technol 30:1687–1698
Tan XL, Wang XK, Geckeis H, Rabung TH (2008) Environ Sci Technol 42:6532–6537
Tao ZY, Zhang J, Zhai JJ (1999) Anal Chim Acta 395:199–203
Zhang J, Zhai JJ, Zhao FZ, Tao ZY (1999) Anal Chim Acta 378:177–182
Chin YP, Alken G, O’Loughlin E (1994) Environ Sci Technol 28:1853–1858
Walanda DK, Lawrance GA, Donne SW (2005) J Power Sources 139:325–341
Ananth MV, Pethkar S, Dakshinamurthi K (1998) J Power Sources 75:278–282
Xu M, Kong L, Zhou W, Li H (2007) J Phys Chem C 111:19141–19147
Fei JB, Cui Y, Yan XH, Qi W, Yang Y, Wang KW, He Q, Li JB (2008) Adv Mater. 20:452–456
Li BX, Rong GX, Xie Y, Huang LF, Feng CQ (2006) Inorg Chem 45:6404–6410
Benguella B, Benaissa H (2002) Water Res 36:2463–2474
Tan LQ, Jin YL, Chen J, Cheng XC, Wu J, Feng LD (2011) J Radioanal Nucl Chem 289:601–610
Yüzer H, Kara M, Sabah E, Çelik MS (2008) J Hazard Mater 151:33–37
Wu CH (2007) J Colloid Interface Sci 311:338–346
Pan G, Qin YW, Li XL, Hu TD, Wu ZY, Xie YN (2004) J Colloid Interface Sci 271:28–34
Li XL, Pan G, Qin YW, Hu TD, Wu ZY, Xie YN (2004) J Colloid Interface Sci 27:35–40
Hayes KF, Leckie JO (1987) J Colloid Interface Sci 115:564–572
Matis KA, Zouboulis AI, Hancock IC (1994) Bioresour Technol 49:253–259
Tan XL, Wang XK, Chen CL, Sun AH (2007) Appl Radiat Isot 65:375–381
Cotton F, Wilkinson G (1980) Wiley, New York, pp 255
Esmadi F, Simm J (1995) Colloid Surf A 104:265–270
Chen CL, Wang XK, Jiang H, Hu WP (2007) Colloid Surf A 302:121–125
Hu BW, Cheng W, Zhang H, Sheng GD (2010) J Radioanal Nucl Chem 285:389–398
Langmuir I (1918) J Am Chem Soc 40:1361–1403
Kilpatrick M, Baker Jr, McKinney LL, Jr CD (1953) J Phys Chem C 57:385–390
Zhou YT, Nie HL, Branford-White C, He ZY, Zhu LM (2009) J Colloid Interface Sci 330:29–37
Ajmal M, Rao RAK, Anwar S, Ahmad J, Ahmad R (2003) Bioresour Technol 86:147–149
Acknowledgment
This work was financially supported by the Award Fund for Prominent Youth Scientists of Shandong Province of China (Project No. BS 2009DX014).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mou, J., Wang, G., Shi, W. et al. Sorption of radiocobalt on a novel γ-MnO2 hollow structure: effects of pH, ionic strength, humic substances and temperature. J Radioanal Nucl Chem 292, 293–303 (2012). https://doi.org/10.1007/s10967-011-1408-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-011-1408-0