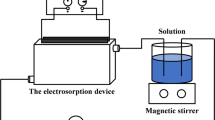
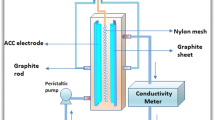
Abstract
A study on the electrosorption of uranium (U(VI)) ions onto a porous activated carbon fiber was performed to treat lagoon sludge containing 100 mg/L uranium and high concentration of chemical salts composed 3.8% NaNO3, 19.8% NH4NO3, 1.9% Ca(NO3)2. The applied negative potential increased the adsorption kinetics and capacity in comparison to the open-circuit potential adsorption for uranium ions. When applying potential at −0.9 V (vs. Ag/AgCl) and pH 4, above 99% of the uranium is selectively removed from the 100 mg/L influent by electrosorption, and the cumulative amount of uranium for 50 h is about 600 mguranium/gACF. The high selectivity of elctrosorption process for uranium was probably caused by the difference of charge density of cations. More than 99% of adsorbed uranium ions was desorbed at a potential of +1.2 V and pH 3. The electrosorption of uranium onto the porous activated carbon fiber electrode is due to an ion exchange type reaction between the uranium ions and surface acid groups on carbon surface. Cyclic electrosorption test consisting of adsorption and desorption step shows that the activated carbon fiber electrode is easily regenerated in situ, indicating it is a reversible process.










Similar content being viewed by others
References
Kaplan D, Gervais T, Krupka K (1998) Radiochim Acta 80:201–211
Konstatinou M, Pashalidis I (2007) J Radioanal Nucl Chem 273:549–552
Sato T, Murakamit T, Yanase N, Isobe H, Payne T, Airey P (1997) Environ Sci Technol 31:2854–2858
Guler H, Sahiner G, Aycik A, Guven O (1997) J Appl Polym Sci 66:2475–2480
Wei Y, Kumagai M, Takashima Y, Asou M, Namba T, Suzuki K, Maekawa A, Ohe S (1998) J Nucl Sci Technol 35:357–364
Nakajima A, Sakaguchi T (1999) J Radioanal Nucl Chem 242:623–626
Inoue K, Kawakita H, Ohto K, Oshima T, Murakami H (2006) J Radioanal Nucl Chem 267:435–442
Donat R, Aytas S (2005) J Radioanal Nucl Chem 265:107–114
Donat R, Esen K, Cetisli H, Aytas S (2009) J Radioanal Nucl Chem 279:253–261
Sauer N, Smith B (1993) Metal-ion recycle technology for metal electroplating waste waters. LA-12532-MS
Hazourli S, Bonnecaze G, Astruc M (1996) Adsorption and electrosorption of organic compounds on granular activated carbon. Environ Technol 17:1275–1283
Jayson G, Sangster J, Thompson G, Wilkinson M (1987) Carbon 25:523–531
Carley-Macauy K, Gutman R (1981) Radioactive waste: advanced management methods for medium active liquid waste. Harwood Academic Publishers, Brussels
Gabelich C, Tran T, Suffet I (2002) Environ Sci Technol 36:3010
Trainham J, Newman J (1997) J Electrochem Soc 124:1528–1540
Oren Y, Soffer A (1983) Electrochim Acta 28:1649–1654
Hou C, Patricia T, Sotira Y, Costas T (2008) J Chem Phys 129:224703
Shmidt J, Pimenov A, Lieberman A, Chen H (1997) Sep Sci Technol 32:2105–2121
Defay R, Prigogine I, Bellemans A, Everett D (1970) Surface tension and adsorption. Longmlans, London
Tomizuka I, Meguro T, Kuwagaki H, Chiba A, Miyazaki A, Okamoto M (1994) Elimination behavior of metallic ion from its dilute solution by activated carbon fiber under applied electrical potential. In: Proceeding of Carbon ’94, Granada, Spain
Hatfield T, Kleven T, Pierce D (1996) J Appl Electrochem 26:567–574
Evans S (1966) J Electrochem Soc 113:2975
Seron A, Benaddi H, Beguin F, Frackowiak E, Bretelle J, Thiry M, Bandosz T, Jagiello J, Schwarz J (1996) Carbon 34:481–487
Lange N (2004) Lange’s handbook of chemistry. McGraw-Hill, New York
Acknowledgment
This work has been carried out under the Nuclear R & D Program funded by the Ministry of Education, Science and Technology of Korea.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jung, CH., Lee, HY., Moon, JK. et al. Electrosorption of uranium ions on activated carbon fibers. J Radioanal Nucl Chem 287, 833–839 (2011). https://doi.org/10.1007/s10967-010-0848-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-010-0848-2