Abstract
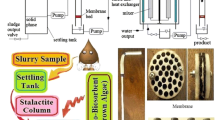
Composites could be more effective adsorbents than inorganic and organic components individually. In the present study, the red macro marine algae, Jania Rubens and yeast, Saccharomyces cerevisiae immobilized on silica gel were used as a constituent of bi-functionalized biosorbent to remove thorium ions from aqueous solution. Optimum biosorption conditions were determined as a function of pH, initial Th(IV) concentration, contact time, temperature, volume/mass ratio and co-ion effect. The morphological analysis of the biocomposite was performed by the scanning electron microscopy and functional groups in the biosorbent were determined by FT-IR spectroscopy. In order to find the adsorption characteristics, Langmuir, Freundlich, and Dubinin–Radushkevich adsorption isotherms were applied to the adsorption data. The data were well described by Langmuir adsorption isotherms while the fit of Freundlich adsorption isotherms and Dubinin–Radushkevich equation to adsorption data was poor. Using the equilibrium constant value obtained at different temperature, the thermodynamics properties of the biosorption (ΔG°, ΔH° and ΔS°) were also determined. The results show that biosorption of Th(IV) ions onto biocomposite was exothermic nature, spontaneous and more favorable at lower temperature under examined conditions.










Similar content being viewed by others
Abbreviations
- b :
-
Langmuir constants related to sorption energy (L/mg)
- C ads :
-
concentration of metal sorbed onto adsorbent (mg/g)
- C e :
-
equilibrium concentration of metal in solution (mg/L)
- C i :
-
the concentrations of the thorium in initial solution (mg/L)
- C f :
-
the concentrations of the thorium in final solution (mg/L)
- E :
-
sorption energy (kJ/mol)
- K d :
-
distribution coefficient (mL/g)
- K :
-
empirical constants of Freundlich isotherm (mg/g)
- m :
-
the weight of biocomposite (g)
- n :
-
empirical constants of Freundlich isotherm
- Q 0 :
-
Langmuir constants related to sorption capacity (mg/g)
- q e :
-
amount of metal ions sorbed onto adsorbent (mg/g)
- R :
-
universal gas constant (J/mol K)
- T :
-
solution temperature (K)
- V:
-
volume of the aqueous phase (mL)
- X m :
-
the maximum sorption capacity of D-R isotherm (mmol/g)
- β :
-
constant related to biosorption energy (mol/J)2
- ε :
-
the Polanyi potential (kJ/mol)
- ∆H°:
-
the change in enthalpy (kJ/mol)
- ∆S°:
-
the change in entropy (kJ/mol K)
- ΔG°:
-
Gibbs free energy change (kJ/mol)
References
Mungasavalli DP, Viraraghavan T, Jin YC (2007) Biosorption of chromium from aqueous solutions by pretreated Aspergillus niger: batch and column studies. Colloids Surf A Physicochem Eng Aspects 301:214–223
Spinti M, Zhuang H, Trujillo EM (1995) Evaluation of immobilized biomass beads for removing heavy metals from wastewaters. Water Environ Res 67:943–952
Srinath T, Verma T, Ramteke PW, Garg SK (2002) Chromium(VI) biosorption and bioaccumulation by chromate resistant bacteria. Chemosphere 48:427–435
Holan ZR, Volesky B (1995) Biosorption of heavy metals. Biotechnol Prog 11:235–250
Gardea-Torresdey JL, Becker-Hapak MK, Hosea JM, Darnall DW (1990) Effect of chemical modification of algal carboxyl groups on metal ion binding. Environ Sci Technol 24:1372–1378
Volesky B (1991) Biosorption of heavy metals. CRC Press, Boca Raton
Schiewer S, Volesky B (2000) Biosorption processes for heavy metal removal. In: Lovley DR (ed) Environmental microbe–metal interactions. ASM Press, Washington, DC
Crist RH, Martin JR, Carr D, Watson JR, Clarke HJ (1994) Interactions of metals Ni2+, Cd2+, Ca2+, Mg2+, Cu2+, Pb2+, Zn2+) and protons with algae. 4. Ion exchange vs. adsorption models and a reassessment of Scatchard plots; ion exchange rates and equilibrium compared with calcium alginate. Environ Sci Technol 28:1859–1866
Schiewer S, Volesky B (1995) Modeling of the proton metal ion exchange in biosorption. Environ Sci Technol 29:3049–3058
Schiewer S, Volesky B (1996) Modeling multi-metal ion exchange in biosorption. Environ Sci Technol 30:2921–2927
Wang J, Chen C (2009) Biosorbent for heavy metals removal and their future. Biotechnol Adv 27:195–226
Sarı A, Tuzen M (2008) Biosorption of total chromium from aqueous solution by red algae (Ceramium virgatum): equilibrium, kinetic and thermodynamic studies. J Hazard Mater 160:349–355
Mahmoud ME, Yakout AA, Osman MM (2009) Dowex anion exchanger-loaded-baker’s yeast as bi-functionalized biosorbents for selective extraction of anionic and cationic mercury(II) species. J Hazard Mater 164:1036–1044
Wang J, Chen C (2006) Biosorption of heavy metals by Saccharomyces cerevisiae: a review. Biotechnol Adv 24:427–451
Rangsayatorn N, Pokethitiyook P, Upatham ES, Lanza GR (2004) Cadmium biosorption by cells of Spirulina platensis TISTR 8217 immobilized in alginate and silica gel. Environ Int 30:57–63
Marseaut S, Debourg A, Dostálek P, Votruba J, Kuncová G, Tobin JM (2004) A silica matrix biosorbent of cadmium. Int Biodeter Biodegr 54:209–214
Ngeontae W, Aeungmaitrepirom W, Tuntulani T (2007) Chemically modified silica gel with aminothioamidoanthraquinone for solid phase extraction and preconcentration of Pb(II), Cu(II), Ni(II), Co(II) and Cd(II). Talanta 71:1075–1082
Chaiko D, Kopasz JP, Ellison AJG (1998) Use of sol–gel systems for solid liquid separation. Ind Eng Chem Res 37:1071–1078
Cestari AR, Airoldi C (1997) Chemisorption on Thiol–Silicas: Divalent Cations as a Function of pH and Primary Amines on Thiol–Mercury Adsorbed. J Colloid Interface Sci 195:338–347
Akar T, Kaynak Z, Ulusoy S, Yuvaci D, Ozsari G, Akar ST (2009) Enhanced biosorption of nickel(II) ions by silica-gel-immobilized waste biomass: biosorption characteristics in batch and dynamic flow mode. J Hazard Mater 163:1134–1141
Bağ H, Lale M, Türker AR (1998) Determination of iron and nickel by flame atomic absorption spectrophotometry after preconcentration on Saccharomyces cerevisiae immobilized sepiolite. Talanta 47:689–696
Eisenbud M (1987) Environmental radioactivity from natural industrial and military sources. Academic Press, San Diego
Macaskie LE (1991) The application of biotechnology of the treatment of wastes produced from the nuclear fuel cycle: biodegradation and bioaccumulation as a means of treating radionuclide-containing streams. Crit Rev Biotechnol 11:41–112
Tsezos M, Volesky B (1981) Biosorption of uranium and thorium. Biotechnol Bioeng 23:583–604
Ryabchikov DI, Gol’braikh EK (1963) The analytical chemistry of Thorium. Pergamon Press, Oxford
Bhainsa KC, D’Souza SF (2009) Thorium biosorption by Aspergillus fumigatus, a filamentous fungal biomass. J Hazard Mater 165:670–676
Humelnicu D, Drochioiu G, Popa K (2004) Bioaccumulation of thorium and uranyl ions on Saccharomyces cerevisiae. J Radioanal Nucl Chem 260:291–293
Liao X, Li L, Shi B (2004) Adsorption recovery of thorium(IV) by Myrica rubra tanin and larch tannin immobilized onto collagen fibres. J Radioanal Nucl Chem 260:619–625
Aslani MAA, Eral M, Akyil S (1998) Separation of thorium from aqueous solution using silk fibroin. J Radioanal Nucl Chem 238:123–127
Andres Y, MacCordick HJ, Hubert JC (1992) Bacterial biosorption and retention of thorium and uranyl cations by Mycobacterium smegmatis. J Radioanal Nucl Chem 166:431–440
Bursali EA, Merdivan M, Yurdakoc M (2010) Preconcentration of uranium(VI) and thorium(IV) from aqueous solutions using low-cost abundantly available sorbent. J Radioanal Nucl Chem 283:471–476
Inoue K, Kawakita H, Ohto K, Oshima T, Murakami H (2006) Adsorptive removal of uranium and thorium with a crosslinked persimmon peel gel. J Radioanal Nucl Chem 267:435–442
Salinas-Pedroza MG, Olgun MT (2004) Thorium removal from aqueous solutions of Mexican erionite and X zeolite. J Radioanal Nucl Chem 260:115–118
Donat R, Aytas S (2005) Adsorption and thermodynamic behavior of uranium(VI) on Ulva sp.-Na bentonite composite adsorbent. J Radioanal Nucl Chem 265:107–114
Donat R, Cilgi GK, Aytas S, Cetisli H (2009) Thermodynamic parameters and sorption of U(VI) on ACSD. J Radioanal Nucl Chem 279:271–280
Donat R, Esen K, Cetisli H, Aytas S (2009) Adsorption of uranium(VI) onto Ulva sp.-sepiolite composite. J Radioanal Nucl Chem 279:253–261
Mahan CA, Holcombe P (1992) Immobilization of algae cells on silica gel and their characterization for trace metal preconcentration. Anal Chem 64:1933–1939
Sheng PX, Ting YP, Chen JP, Hong L (2004) Sorption of lead, copper, cadmium, zinc and nickel by marine algal biomass. J Colloid Interface 275:131–141
Pagnanelli F, Petrangeli PM, Toro L, Trifoni M, Veglio F (2000) Biosorption of metal ions on Arthrobacter sp.: biomass characterization and biosorption modeling. Environ Sci Technol 34:2773–2778
Beech I, Hanjagsit L, Kalaji M, Neal AL, Zinkevich V (1999) Chemical, structural characterization of exopolymers produced by Pseudomonas sp. NCIMB 2021 in continuous culture. Microbiology 145:1491–1497
Kazy SK, Sar P, Sen AK, Singh SP, D’Souza SF (2002) Extracellular polysaccharides of a copper-sensitive and a copper-resistant Pseudomonas aeruginosa strain: synthesis, chemical nature and copper binding. W J Microbiol Biotechnol 18:583–588
Kaygun AK, Akyil S (2007) Study of the behaviour of thorium adsorption on PAN/zeolite composite adsorbent. J Hazard Mater 147:357–362
Bayramoglu G, Celik G, Arica MY (2006) Studies on accumulation of uranium by fungus Lentnus sajor-caju. J Hazard Mater 136:345–1345
Bhat SV, Melo JS, Chaugule BB, D’Souza SF (2008) Biosorption characteristics of uranium (VI) from aqueous medium onto Catenella repens, a red alga. J Hazard Mater 158:628–635
Aytas S, Yurtlu M, Donat R (2009) Adsorption characteristic of U(VI) ion onto thermally activated bentonite. J Hazard Mater 172:667–674
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinium. J Am Chem Soc 40:1361–1403
Freundlich HMF (1906) Über die adsorption in lösungen. Z Physikalische Chem (Leipzig) 57A:385–470
Khan SA, Rehman R, Khan MA (1995) Adsorpton of chromium (III), chromium (VI) and silver (I) on bentonite. Waste Manage 15:271–282
Donat R (2009) The removal of uranium (VI) from aqueous solutions onto natural sepiolite. J Chem Thermodyn 41:829–835
Acknowledgments
Cem Gök would like to acknowledge The Scientific and Technological Research Council of Turkey (TUBITAK) for providing necessary fellowships. The authors also would like to express sincere thanks to Assoc.Prof.Dr. M.A.A. Aslani, Prof.Dr. S. Akyıl Erentürk and Ress. Assist. S. Doyurum for their helps and supports.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gok, C., Turkozu, D.A. & Aytas, S. Removal of Th(IV) ions from aqueous solution using bi-functionalized algae-yeast biosorbent. J Radioanal Nucl Chem 287, 533–541 (2011). https://doi.org/10.1007/s10967-010-0788-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-010-0788-x