Summary
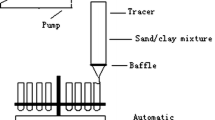
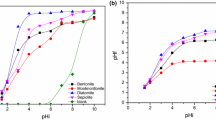
The adsorption kinetics of strontium ion was studied on seven natural clay samples with radioactive tracer method. The kinetic curves were determined and the kinetic data were evaluated by forms of first-rate kinetic equations with different terms, generally used for adsorption of ions of low concentration. The adsorption process was reduced to two steps. Film diffusion and participle diffusion were found in the case of five samples. Gel diffusion, film diffusion and participle diffusion were found in the case of the other two samples. The presence of significant amount of cristobalite can explain the gel phase in these two samples. The rate coefficients of steps were calculated from the kinetic curves.
Similar content being viewed by others
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nemes, Z., Nagy, N. & Kónya, J. Kinetics of strontium ion adsorption on natural clay samples. J Radioanal Nucl Chem 266, 289–293 (2005). https://doi.org/10.1007/s10967-005-0906-3
Issue Date:
DOI: https://doi.org/10.1007/s10967-005-0906-3