Abstract
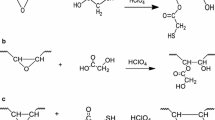
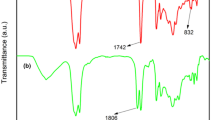
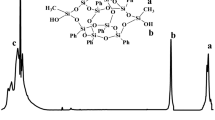
A synthetic strategy for the preparation of novel and reprocessable biobased polyisobutylene (PIB) – polyurethane (PU) network with soybean oil (SBO) derivative was reported. For this purpose, SBO triglycerides were converted to a polyol with primary hydroxyl functionalities via UV activated thiol-ene chemistry. Optimization of grafting of hydroxyl groups was performed by varying the process parameters such as ratios of both initiator and thiol to double bond as well as the irradiation time. SBO/PIB based PU network (X-SBO/PIB-PU) was then prepared by successful integration of the SBO based polyol to PIB-PU formulation as a co-polyol. The resultant network formed a nearly optically clear film which can be swollen in good solvent. Selective cleavage of the ester bonds by acid catalyzed hydrolysis was found to be an efficient way to solubilize the network by enabling the recovery of polyurethane segments with carboxylic acid functionalities. Thermal, mechanical and microstructural characterization data confirmed that hydrolysis of the network can be conducted without disturbing the polyurethane structures with high mechanical and thermal stability. It has been suggested that hydrolyzed X-SBO/PIB-PU (H-SBO/PIB-PU) with polar functional groups can then be used as building block in the development of polymer conjugates formulated with rigid polymers and nanocomposites.















Similar content being viewed by others
Data availability
The data that supports the findings of this study are available from the corresponding author upon the reasonable request.
References
Prisacariu C (2011) Polyurethane elastomers. From morphology to mechanical aspects. Springer, Vienna
Heath DE, Cooper SL (2013) In: Ratner BD, Hoffman AS, Schoen FJ, Lemons JE (eds) Biomaterials science. An introduction to materials in medicine, 3rd edn. Academic Press
Deodhar T, Nugay N, Nugay T, Patel R, Moggridge G, Kennedy JP (2023) Synthesis of high molecuar weight and strength polyisobutylene-based polyurethane and its use for the development of a synthetic heart valve. Macromol Rapid Commun 44:2200147
Venkateshaiah A, Padil VVT, Nagalakshmaiah M, Waclawek S, Cernik M, Varma RS (2020) Microscopic techniques for the analysis of micro and nanostructures of biopolymers and their derivatives. Polymers 12:512
Chaffin KA, Buckalew AJ, Schley JL, Chen X, Jolly M, Alkatout JA, Miller JP, Untereker DF, Hillmyer MA, Bates FS (2012) Influence of water on the structure and properties of PDMS containing multiblock polyurethanes. Macromolecules 45:9110–9120
Chaffin KA, Chen X, McNamara L, Bates FS, Hillmyer MA (2014) Polyether urethane hydrolytic stability after exposure to deoxygenated water. Macromolecules 47:5220–5226
Crago M, Lee A, Farajikhah S, Oveissi F, Fletcher DF, Dehghani F, Winlaw DS, Naficy S (2022) The evolution of polyurethane heart valve replacements: How chemistry translates to the clinic. Mater Today Commun 33:104916
Kang J, Erdodi G, Kennedy JP (2011) Polyisobutylene-based polyurethanes with unprecedented properties and how they came about. J Polym Sci Part A Polym Chem 49:3891–3904
Odian G (2004) Principles of Polymerization. John Wiley and Sons, Hoboken, New Jersey
Pinchuk L, Wilson GJ, Barry JJ, Schoephoerster RT, Parel JM, Kennedy JP (2008) Medical applications of poly(styrene-block-isobutylene-block-styrene)(“SIBS”)). Biomaterials 29:448–460
Kang J, Erdodi G, Brendel CM, Ely D, Kennedy JP (2010) Polyisobutylene-based polyurethanes. V. Oxidative-hydrolytic stability and biocompatibility. J Polym Sci Part A Polym Chem 48:2194–2203
Kang J, Kennedy JP (2015) Hydrolytically stable polyurethanes. J Polym Sci Part A Polym Chem 53:1–4
Toth K, Nugay N, Kennedy JP (2016) Polyisobutylene-based polyurethanes: VII. Structure/property investigations for medical applications. J Polym Sci Part A Polym Chem 54:532–543
Kekec NC, Akolpoglu MB, Bozuyuk U, Kizilel S, Nugay N, Nugay T, Kennedy JP (2019) Calcification resistance of polyisobutylene and polyisobutylene-based materials. Polym Adv Technol 30:1836–1846
Senel Kekec NC (2023) High strength polyisobutylene based structures: synthesis, characterization and suitability for biomedical applications. Doctoral dissertation, Bogazici University
Weldels S, Averous L (2021) Biobased polyurethanes for biomedical applications. Bioact Mater 6:1083–1106
Pechar TW, Sohn S, Wilkes GL, Ghosh S, Frazier CE, Fornof A, Long TE (2006) Characterization and comparison of polyurethane networks prepared using soybean-based polyols with varying hydroxyl content and their blends with petroleum-based polyols. J Appl Polym Sci 101:1432–1443
Robertson ML, Hillmyer MA, Mortamet AC, Ryan AJ (2010) Biorenewable Multiphase Polymers. MRS Bull 35:194–200
Morales-Cerrada R, Tavernier R, Caillol S (2021) Fully Bio-Based Thermosetting Polyurethanes from Bio-Based Polyols and Isocyanates. Polymers 13(8):1255
Kennedy JP, Erdodi G, Jewrajka S (2010) Polymers having both hard and soft segments, and process for making same, WO 2010/039986 A1
Murgasova R, Brantley EL, Hercules DM, Nefzger H (2002) Characterization of polyester-polyurethane soft and hard blocks by a combination of MALDI, SEC, and chemical degradation. Macromolecules 35:8338–8345
Chapman TM (1989) Models for polyurethane hydrolysis under moderately acidic conditions: a comparative study of hydrolysis rates of urethanes, ureas, and amides. J Polym Sci Part A Polym Chem 27:1993–2005
Erman B, Baysal BM (1985) Temperature dependence of swelling of polystyrene networks. Macromolecules 18:1696–1700
Sugiyama F, Kleinschmidt AT, Kayser LV, Rodriquez D, Finn M, Alkhadra MA, Wan JMH, Ramírez J, Chiang ASC, Root SE, Savagatrupa S, Lipomi DJ (2018) Effects of flexibility and branching of side chains on the mechanical properties of low-bandgap conjugated polymer. Polym Chem 9:4354
Meier MAR, Metzger JO, Schubert US (2007) Plant oil renewable resources as green alternatives in polymer science. Chem Soc Rev 36:1788–1802
Pfister DP, Xia Y, Larock RC (2011) Recent advances in vegetable oil-based polyurethanes. Chemsuschem 4:703–717
Lligadas G, Ronda JC, Galia M, Cadiz V (2010) Plant oils as platform chemicals for polyurethane synthesis: Current state-of-the-art. Biomacromol 11:2825–2835
Xia Y, Larock RC (2010) Vegetable oil-based polymeric materials: synthesis, properties, and applications. Green Chem 12:1893–1909
Petrovic ZS (2008) Polyurethanes from vegetable oils. Polym Rev 48:109–155
Desroches M, Escouvois M, Auvergne R, Caillol S, Boutevin B (2012) From vegetable oils to polyurethanes: Synthetic routes to polyols and main industrial products. Polym Rev 52:38–79
Sawpan AM (2018) Polyurethanes from vegetable oils and applications-a review. J Polym Res 25:184
Rahman MZ, Rahman M, Mahbub T, Ashiquzzaman M, Sagadevan S, Hoque ME (2023) Advanced biopolymers for automobile and aviation engineering applications. J Polym Res 30:106
Alagi P, Ghorpade R, Jang JH, Patil C, Jirimali H, Gite V, Hong SC (2018) Functional soybean oil-based polyols as sustainable feedstocks for polyurethane coatings. Ind Crops Prod 113:249–258
Desroches M, Caillol S, Lapinte V, Auvergne R, Boutevin B (2011) Synthesis of biobased polyols by thiol-ene coupling from vegetable oils. Macromolecules 44:2489–2500
Caillol S, Desroches M, Carlotti S, Auvergne R, Boutevin B (2013) Synthesis of new polyurethanes from vegetable oil by thiol-ene coupling. Green Mater 1:16–26
Alagi P, Choi YJ, Seog J, Hong SC (2016) Efficient and quantitative chemical transformation of vegetable oils topolyols through a thiol-ene reaction for thermoplastic polyurethanes. Ind Crops Prod 87:78–88
Alagi P, Choi YJ, Hong SC (2016) Preparation of vegetable oil-based polyols with controlled hydroxyl functionalities for thermoplastic polyurethane. Eur Polym J 78:46–60
Samuelsson J, Jonsson M, Brinck T, Johansson M (2004) Thiol–ene coupling reaction of fatty acid monomers. J Polym Sci Part A Polym Chem 42:6346–6352
Turunc O, Meier MAR (2010) Fatty acid derived monomers and related polymers via thiol-ene (click) additions. Macromol Rapid Commun 31:1822–1826
Lluch C, Ronda JC, Galia M, Lligadas G, Cadiz V (2010) Rapid approach to biobased telechelics through two one-pot thiol-ene click reactions. Biomacromol 11:1646–1653
Desroches M, Caillol S, Auvergne R, Boutevin B, David G (2012) Biobased cross-linked polyurethanes obtained from ester/amide pseudo-diols of fatty acid derivatives synthesized by thiol–ene coupling. Polym Chem 3:450–457
Pham PD, Lapinte V, Raoul Y, Robin JJ (2014) Lipidic polyols using thiol-ene/yne strategy for crosslinked polyurethanes J Polym Sci Part A Polym Chem 52:1597–1606
Barison A, da Silva CW, Campos FR, Simonelli F, Lenz CA, Ferreira AG (2010) A simple methodology for the determination of fatty acid composition in edible oils through 1H NMR spectroscopy. Magn Reson Chem 48:642–650
Vlachos N, Skopelitis Y, Psaroudaki M, Konstantinidou V, Chatzilazarou A, Tegou E (2006) Applications of fourier transform-infrared spectroscopy to edible oils. Anal Chim Acta 573–574:459–465
Zhang Q, Saleh ASM, Shen Q (2016) Monitoring of changes in composition of soybean oil during deep-fat frying with different food types. J Am Oil Chem Soc 93:69–81
Poiana MA, Alexa E, Munteanu MF, Gligor R, Moigradean D, Mateescu C (2015) Use of ATR-FTIR spectroscopy to detect the changes in extra virgin olive oil by adulteration with soybean oil and high temperature heat treatment. Open Chem 13:689–698
Srichatrapimuk VW, Cooper SL (1978) Infrared thermal analysis of polyurethane block polymers. J Macromol Sci Part B Phys 15:267–311
Walch E, Gaymans RJ (1994) Telechelic polyisobutylene with unsaturated end groups and with anhydride end groups. Polymer 35:1774–1778
Socrates G (2001) Infrared and raman characteristic group frequencies tables and charts, 3rd edn. Wiley, Chichester
Lu QW, Hoye TR, Macosko CW (2002) Reactivity of common functional groups with urethanes: Models for reactive compatibilization of thermoplastic polyurethane blends. J Polym Sci Part A Polym Chem 40:2310–2328
Toth K, Kekec NC, Nugay N, Kennedy JP (2016) Polyisobutylene-based polyurethanes. VIII. Polyurethanes with -O-S-PIB-S-O- soft segments. J Polym Sci Part A Polym Chem 54:1119–1131
Lligadas G, Ronda JC, Galià M, Biermann U, Metzger JO (2006) Synthesis and characterization of polyurethanes from epoxidized methyl oleate based polyether polyols as renewable resources. J Polym Sci Part A Polym Chem 44:634
Nugay N, Nugay T, Deodhar T, Keszler BL, Kennedy JP (2018) Low cost bifunctional initiators for bidirectional living cationic polymerization of olefins. II. Hyperbranched styrene–isobutylene–styrene triblocks with superior combination of properties. J Polym Sci Part A Polym Chem 56:705–713
Hu L, Pu Z, Zhong Y, Liu L, Cheng J, Zhong J (2020) Effect of different carboxylic acid group contents on microstructure and properties of waterborne polyurethane dispersions. J Polym Res 27:129
Levchik GF, Si K, Levchik SV, Camino G, Wilkie CA (1999) The correlation between cross-linking and thermal stability: Cross-linked polystyrenes and polymethacrylates. Polym Degrad Stab 65:395–403
Mittal N, Benselfelt T, Ansari F, Gordeyeva K, Roth SV, Wagberg L, Söderberg LD (2019) Ion-specific assembly of strong, tough, and stiff biofibers. Angew Chem Int 58:18562–18569
Funding
The authors thank Bogazici University Research Foundation Projects (project 11763) for financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kurnaz, E., Şen, S., Nugay, N. et al. Biobased reprocessable polyisobutylene - polyurethane networks. J Polym Res 30, 327 (2023). https://doi.org/10.1007/s10965-023-03715-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-023-03715-5