Abstract
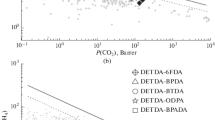
This work reported the gas permeation performances of three 6FDA-based polyimides, including 6FDA-DABA, 6FDA-ODA and 6FDA-TFDB, which contain polar amide, flexible ether, and bulky –CF3 groups in diamine units, respectively. The effect of diamine units on the microstructure and gas transmission performance of polyimides was detailly researched via positron annihilation, X-ray diffractogram and molecular simulations, with respect to chain packing, hydrogen bonding, free volumes and movement of molecular chains. As the diamine units changed from amide to ether and –CF3, the gas transport coefficient of polyimides enhanced, and the gas transport selectivity (including H2/N2, O2/N2, CO2/CH4 and CO2/N2) decreased. With the introduction of amide to diamine moieties, a large number of hydrogen bonds were formed in 6FDA-DABA, which improved the chains packing and decreased free volumes and chains movement. Consequently, the 6FDA-DABA showed the lowest permeation coefficients. Bulky –CF3 in the diamine greatly hindered chain stacking, which increased free volume, promoted chains movement and endowed 6FDA-TFDB with the highest permeation coefficients. The decreasing permeation selectivity from 6FDA-DABA to 6FDA-ODA and 6FDA-TFDB was primarily ascribed to the reduction of diffusive selectivity, which was stemmed from the improved free volume and chains mobility.








Similar content being viewed by others
Data availability
The raw data required to reproduce these findings are included in the article and in the Supplementary data file.
References
Deng G, Wang Y, Zong X, Yuan X, Luo J, Wang X, Xue S (2019) Gas transport property of the binaphthyl-based polyimide membranes. Polymer 183:121854. https://doi.org/10.1016/j.polymer.2019.121854
Xiao Y, Low BT, Hosseini SS, Chung TS, Paul DR (2009) The strategies of molecular architecture and modification of polyimide-based membranes for CO2 removal from natural gas-a review. Prog Polym Sci 34:561–580. https://doi.org/10.1016/j.progpolymsci.2008.12.004
Wu AX, Drayton JA, Rodriguez KM, Qian Q, Lin S, Smith ZP (2020) Influence of aliphatic and aromatic fluorine groups on gas permeability and morphology of Fluorinated Polyimide Films. Macromolecules 53:5085–5095. https://doi.org/10.1021/acs.macromol.0c01024
Favvas EP, Katsaros FK, Papageorgiou SK, Sapalidis AA, Mitropoulos AC (2017) A review of the latest development of polyimide based membranes for CO2 separations. React Funct Polym 120:104–130. https://doi.org/10.1016/j.reactfunctpolym.2017.09.002
Robeson LM (1991) Correlation of separation factor versus permeability for polymeric membranes. J Membrane Sci 62:165–185. https://doi.org/10.1016/0376-7388(91)80060-J
Yahay GO, Mokhtari I, Alghannam AA, Choi SH, Maab H, Bahamdan AA (2018) Cardo-type random co-polyimide membranes for high pressure pure and mixed sour gas feed separations. J Membrane Sci 550:526–535. https://doi.org/10.1016/j.memsci.2017.10.063
Plaza-Lozano D, Comesaña-Gándara B, de la Viuda M, Seong JG, Palacio L, Prádanos P, de la Campa JG, Cuadrado P, Lee YM, Hernández A, Alvarez C, Lozano AE (2015) New aromatic polyamides and polyimides having an adamantane bulky group. Mater Today Commum 5:23–31. https://doi.org/10.1016/j.mtcomm.2015.10.001
Zhuang Y, Seong JG, Do YS, Jo JH, Cui Z, Lee J, Lee YM, Guiver MD (2014) Intrinsically microporous soluble polyimides incorporating Tröger’s base for membrane gas separation. Macromolecules 47:3254–3262. https://doi.org/10.1021/ma5007073
Carta M, Croad M, Malpass-Evans R, Jansen JC, Bernardo P, Clarizia G, Friess K, Lanc M, McKeown NB (2014) Triptycene Induced enhancement of membrane gas selectivity for Microporous Tröger’s base polymers. Adv Mater 26:3526–3531. https://doi.org/10.1002/adma.201305783
Álvarez C, Lozano AL, de la José G (2016) High-productivity gas separation membranes derived from pyromellitic dianhydride and nonlinear diamines. J Membrane Sci 501:191–198. https://doi.org/10.1016/j.memsci.2015.11.039
Rojas-Rodríguez M, Aguilar-Lugo C, Lozano AE, Hernández A, Mancilla-Cetina E, Alexandrova L (2020) Synthesis and properties of highly processable asymmetric polyimides with bulky phenoxy groups. High Perform Polym 32:455–468. https://doi.org/10.1177/0954008319877455
Álvarez C, Lozano AE, Juan-y-Seva M, José G, de la Campa (2020) Gas separation properties of aromatic polyimides with bulky groups. Comparison of experimental and simulated results. J Membrane Sci 602:117959. https://doi.org/10.1016/j.memsci.2020.117959
Abdulhamid MA, Genduso G, Ma X, Pinnau I (2021) Synthesis and characterization of 6FDA/3, 5-diamino-2, 4, 6-trimethylbenzenesulfonic acid-derived polyimide for gas separation applications. Sep Purif Technol 257:117910. https://doi.org/10.1016/j.seppur.2020.117910
Alaslai N, Ghanem B, Alghunaimi F, Pinnau I (2016) High-performance intrinsically microporous dihydroxyl-functionalized triptycene-based polyimide for natural gas separation. Polyme 91:128–135. https://doi.org/10.1016/j.polymer.2016.03.063
Alaslai N, Ghanem B, Alghunaimi F, Litwilleret E, Pinnau I (2016) Pure-and mixed-gas permeation properties of highly selective and plasticization resistant hydroxyl-diamine-based 6FDA polyimides for CO2/CH4 separation. J Membrane Sci 505:100–107. https://doi.org/10.1016/j.memsci.2015.12.053
Abdulhamid MA, Genduso G, Wang Y, Ma X, Pinnau I (2019) Plasticization-resistant carboxyl-functionalized 6FDA-polyimide of intrinsic microporosity (PIM–PI) for membrane-based gas separation. Ind Eng Chem Res 59:5247–5256. https://doi.org/10.1021/acs.iecr.9b04994
Meis D, Neumann S, Filiz V (2022) Thermal rearrangement in thermal cascade reaction polymers via ortho-carbonate ester functionalization of polyimides and their gas separation performance. J Membrane Sci 655. https://doi.org/10.1016/j.memsci.2022.120586
Feng Y, Ren J, Li H, Zhao D, Sheng L, Wu Y, Zhao W, Deng M (2021) Effect of thermal annealing on gas separation performance and aggregation structures of block polyimide membranes. Polymer 219:123538. https://doi.org/10.1016/j.polymer.2021.123538
Liu Z, Qiu W, Quan W, Liu Y, Koros WJ (2021) Fine-tuned thermally cross-linkable 6FDA-based polyimide membranes for aggressive natural gas separation. J Membrane Sci 635. https://doi.org/10.1016/j.memsci.2021.119474
Chang KS, Tung CC, Wang KS, Tung KL (2009) Free volume analysis and gas transport mechanisms of aromatic polyimide membranes: a molecular simulation study. J Phys Chem B 113:9821–9830. https://doi.org/10.1021/jp903551h
Ando S, Sekiguchi K, Mizoroki M, Okada T, Ishige R (2018) Anisotropic linear and volumetric thermal-expansion behaviors of self‐standing polyimide films analyzed by Thermomechanical Analysis (TMA) and optical interferometry. Macromol Chem Phys 219. https://doi.org/10.1002/macp.201700354
Okada T, Ishige R, Ando S (2018) Effects of chain packing and structural isomerism on the anisotropic linear and volumetric thermal expansion behaviors of polyimide films. Polymer 146:386–395. https://doi.org/10.1016/j.polymer.2018.05.059
Wang S, Ma S, He H, Ai W, Wang D, Zhao X, Chen C (2019) Aromatic polyimides containing pyridine and spirocyclic units: preparation, thermal and gas separation properties. Polymer 168:199–208. https://doi.org/10.1016/j.polymer.2019.02.046
Wang C, Zhao X, Tian D, Wang D, Chen C, Zhou H (2017) Synthesis and characterization of novel polyimides derived from 4,4’-bis(5-amino-2-pyridinoxy)benzophenone: effect of pyridine and ketone units in the main. Des Monomers Polym 20:97–105. https://doi.org/10.1080/15685551.2016.1231036
Cottrell T (1958) The strengths of chemical bonds. Butter-worths, London, p 270
Wang R, Falck JR (2013) Transformations of X (C, o, N)-CN bonds: cases of selective X (C, o, N)-C activation. Rsc Adv 4:1062–1066. https://doi.org/10.1039/C3RA45178J
Yang Z, Guo H, Kang C, Gao L (2021) Synthesis and characterization of amide-bridged colorless polyimide films with low CTE and high optical performance for flexible OLED displays. Polym Chem-UK 12:5364–5376. https://doi.org/10.1039/D1PY00762A
Bai L, Zhai L, He MH, Wang CO, Mo S, Fan L (2020) Thermal expansion behavior of poly (amide-imide) films with ultrahigh tensile strength and ultralow CTE. Chin J Polym Sci 38:748–758. https://doi.org/10.1007/s10118-020-2366-1
Kim JY, Cho H, Noh S, Lee Y, Nam YM, Lee C, Jo WH (2012) Charge transport in amorphous low bandgap conjugated polymer/fullerene films. J Appl Phys 111:043710. https://doi.org/10.1063/1.3686633
Parthasarathi R, Bellesia G, Chundawat SPS, Dale BE, Langan P, Gnanakaran S (2011) Insights into Hydrogen Bonding and stacking interactions in cellulose. J Phys Chem A 115:14191–14202. https://doi.org/10.1021/jp203620x
Gospodinova N, Tomšík E (2015) Hydrogen-bonding versus π–π stacking in the design of organic semiconductors: from dyes to oligomers. Prog Polym Sci 43:33–47. https://doi.org/10.1016/j.progpolymsci.2014.10.010
Mattozzi A, Hedenqvist MS, Gedde UW (2007) Diffusivity of n-hexane in poly (ethylene-stat-octene) s assessed by molecular dynamics simulation. Polymer 48:5174–5180. https://doi.org/10.1016/j.polymer.2007.06.051
Hu CC, Chang CS, Ruaan RC, Lai JY (2003) Effect of free volume and sorption on membrane gas transport. J Membrane Sci 226:51–61. https://doi.org/10.1016/j.memsci.2003.07.010
Cho YJ, Park HB (2011) High performance polyimide with high internal free volume elements. Macromol Rapid Comm 32:579–586. https://doi.org/10.1002/marc.201000690
Liu Y, Huang J, Tan JH, Zeng Y, Liu J, Zhang H, Pei Y, Xiang X, Liu Y (2017) Intrinsic high-barrier polyimide with low free volume derived from a novel diamine monomer containing rigid planar moiety. Polymer 114:289–297. https://doi.org/10.1016/j.polymer.2017.03.006
Nagel C, Schmidtke E, Gu1nther-Schade K, Hofmann D, Fritsch D, Strunskus T, Faupel F (2000) Free volume distributions in glassy polymer membranes: comparison between molecular modeling and experiments. Macromolecules 33:2242–2248. https://doi.org/10.1021/ma990760y
Heuchel M, Fritsch D, Budd PM, Mckeown NB, Hofmann D (2008) Atomistic packing model and free volume distribution of a polymer with intrinsic microporosity (PIM-1). J Membrane Sci 318:84–99. https://doi.org/10.1016/j.memsci.2008.02.038
Ronova IA, Rozhkov EM, Alentiev AYA, Yampolskii YP (2003) Occupied and accessible volumes in glassy polymers and their relationship with gas permeation parameters. Macromol Theory Simul 12:425–439. https://doi.org/10.1002/mats.200350002
Liu Y, Tang A, Tan J, Li Y, Wu D, Zhang X, Zhao X, He P, Zhang H (2020) High-barrier polyimide containing fluorenol moiety: gas barrier properties and molecular simulations. React Funct Polym 157:104747. https://doi.org/10.1016/j.reactfunctpolym.2020.104747
Seethamraju S, Ramamurthy PC, Madras G (2013) Ionomer based blend as water vapor barrier material for organic device encapsulation. ACS Appl Mater Inter 5:4409–4416. https://doi.org/10.1021/am4007808
Stern SA (1994) Polymers for gas separations: the next decade. J Membrane Sci 94:1–65. https://doi.org/10.1016/0376-7388(94)00141-3
Seong JG, Zhuang Y, Kim S, Do YS, Lee WH, Guiverc MD, Lee YM (2015) Effect of methanol treatment on gas sorption and transport behavior of intrinsically microporous polyimide membranes incorporating Tröger׳ s base. J Membrane Sci 480:104–114. https://doi.org/10.1016/j.memsci.2015.01.022
Stern SA, Liu Y, Feld WA (1993) Structure/permeability relationships of polyimides with branched or extended diamine moieties. J Polym Sci Pol Phys 31:939–951. https://doi.org/10.1002/polb.1993.090310804
Teplyakov V, Meares P (1990) Correlation aspects of the selective gas permeabilities of polymeric materials and membranes. Gas Sep Purif 4:66–74. https://doi.org/10.1016/0950-4214(90)80030-O
Tan J, Chen C, Liu Y, Wu J, Wu D, Zhang X, He X, She Z, He R, Zhang H (2020) Molecular simulations of gas transport in hydrogenated nitrile butadiene rubber and ethylene–propylene–diene rubber. RSC Adv 10:12475. https://doi.org/10.1039/d0ra00192a
Wang Y, Sheng L, Zhang X, Li J, Wang R (2023) Hybrid carbon molecular sieve membranes having ordered Fe3O4@ZIF-8-derived microporous structure for gas separation. J Membr Sci 666:121127. https://doi.org/10.1016/j.memsci.2022.121127
Acknowledgements
This work was supported by NSAF (U1930203), National Natural Science Foundation of China (No. 52103004), the Natural Science Foundation of Hunan Province (No. 2021JJ50004), the Science Research Project of Hunan Provincial Department of Education (No. 21A0364), the Student Innovation and Entrepreneurship Training Program of China (No. 202111535017) and the Student Innovation and Entrepreneurship Training Program of Hunan Province (No. S202211535096). We would like to thank Prof. Zhiquan Chen from Hubei Nuclear Solid Physics Key Laboratory, Department of Physics, Wuhan University for their help in the measurement of the polymer free volume.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tan, J., Chen, Y., Huang, J. et al. Influence of diamine moieties on the gas permeation performances of polyimide: perspectives from experiment and simulation. J Polym Res 30, 317 (2023). https://doi.org/10.1007/s10965-023-03677-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-023-03677-8