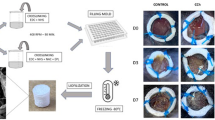
Abstract
The goal of the current study was to evaluate the wound healing potential of electrospun Polycaprolactone—Chitosan—Pectin (PCL-CS-PEC composite nanofiber under in vitro and in vivo conditions in order to determine their appropriateness for its use as wound dressings. 15%, 17%, and 19% PCL-CS-PEC scaffolds were fabricated individually and subjected to various investigations such as hemolysis, swelling, porosity, cell viability, and in-vivo wound healing activity. The collected parameters were subjected to statistical analysis using SPSS version 25 for statistical significance. The current study highlighted that the studied different concentrations of PCL-CS-PEC were hemocompatible since their hemolysis value was found to be less than 5% which is acceptable for any scaffolds or biomaterials universally. The 19% (w/v) PCL-CS-PEC-based scaffold demonstrated the maximum porosity of above 80% across the analyzed concentrations, making it the best choice for tissue engineering applications. The swelling index revealed that 19% of PCL-CS-PEC scaffolds are identified to be superior as it showed 301.7 ± 1.5%, 652.0 ± 2.0%, 902.3 ± 2.1%, and 1151 ± 1.5% for the different time interval of 6,12,18 and 24 h correspondingly than 15% and 17% PCL-CS-PEC nanofiber. Acute dermal toxicity analysis was found to be normal. Wound contraction rate was also found to be superior with 19% PCL-CS-PEC scaffolds than other analyzed concentrations. Our study employed the synthesis of nanofiber with PCL, with an add-on of CS and PEC because PCL has already proven to be wound healing material.







Similar content being viewed by others
References
Guo S, Dipietro LA (2010) Factors affecting wound healing. J Dent Res 89:219–229
Zhu Z, Liu Y, Xue Y, Cheng X, Zhao W, Wang J, He R, Wan Q, Pei X (2019) Tazarotene released from aligned electrospun membrane facilitates cutaneous wound healing by promoting angiogenesis. ACS Appl Mater Interfaces 11:36141–36153
Ghomi ER, Khalili S, Khorasani SN, Neisiany RE, Ramakrishna S (2019) Wound dressings: Current advances and future directions. J Appl Polym Sci 136:47738. https://doi.org/10.1002/app.47738
Tao G, Cai R, Wang Y, Liu L, Zuo H, Zhao P, Umar A, Mao C, Xia Q, He H (2019) Bioinspired design of AgNPs embedded silk sericin-based sponges for efficiently combating bacteria and promoting wound healing. Mater Des 180:107940. https://doi.org/10.1016/j.matdes.2019.107940
Amirian J, Zeng Y, Shekh MI, Sharma G, Stadler FJ, Song J, Du B, Zhu Y (2021) In-situ crosslinked hydrogel based on amidated pectin/oxidized chitosan as potential wound dressing for skin repairing. Carbohydr Polym 251:117005. https://doi.org/10.1016/j.carbpol.2020.117005
Liu X, Xu H, Zhang M, Yu DG (2021) Electrospun medicated nanofibers for wound healing: review membranes (Basel) 11(10):770. https://doi.org/10.3390/membranes11100770
Rosic R, Kocbek P, Pelipenko J, Kristl J, Baumgartner S (2013) Nanofibers and their biomedical use. Acta Pharm 63:295–304
Haider A, Gupta KC, Kang IK (2014) Morphological effects of HA on the cell compatibility of electrospun HA/PLGA composite nanofiber scaffolds. Biomed Res Int 2014:308306. https://doi.org/10.1155/2014/308306
Jiang YN, Mo HY, Yu DG (2012) Electrospun drug-loaded core-sheath PVP/zein nanofibers for biphasic drug release. Int J Pharm 438:232–239
Balaji S, Vaikunth SV, Lang SA, Sheikh AQ, Lim FY, Cromleholme TM, Narmoneva DA (2012) Tissue-engineered provisional matrix as a novel approach to enhance diabetic wound healing. Wound Repair Regen 20:15–27
Uppal R, Ramaswamy GN, Arnold C, Goodband R, Wang Y (2011) Hyaluronic acid nanofiber wound dressing-production, characterization, and in vivo behaviour. J Biomed Mater Res Part B Appl Biomater 97B:20–29
Negm NA, Hefni HHH, Abd-Elaal AAA, Badr EA, Abou Kana MTH (2020) Advancement on modification of chitosan biopolymer and its potential applications. Int J Biol Macromol 152:681–702
Ignatova M, Manolova N, Rashkov I (2013) Electrospun antibacterial chitosan-based fibers. Macromol Biosci 13:860–872
Azimi B, Maleki H, Zavagna L et al (2020) Bio-based electrospun fibers for wound healing. J Funct Biomater 11:1–36
Jayakumar R, Prabaharan M, Kumar PTS, Nair SV, Tamura H (2011) Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol Adv 29:322–337
Dai T, Tanaka M, Huang YY, Hamblin MR (2013) Chitosan preparations for wounds and burns: antimicrobial and wound-healing effects. Expert Rev Anti Infect Ther 9:857–879
Ueno H, Mori T, Fujinaga T (2001) Topical formulations and wound healing applications of chitosan. Adv Drug Deliv Rev 52:105–115
Martins JG, Camargo SEA, Bishop TT, Popat KC, Kipper MJ, Martins AF (2018) Pectin-chitosan membrane scaffold imparts controlled stem cell adhesion and proliferation. Carbohydr Polym 197:47–56
Zahiri M, Khanmohammadi M, Goodarzi A, Ababzadeh S, Sagharjoghi Farahani M, Mohandesnezhad S, Bahrami N, Nabipour I, Ai J (2020) Encapsulation of curcumin loaded chitosan nanoparticle within poly (ε-caprolactone) and gelatin fiber mat for wound healing and layered dermal reconstitution. Int J Biol Macromol 153:1241–1250
Ehterami A, Salehi M, Farzamfar S, Vaez A, Samadian H, Sahrapeyma H, Mirzaii M, Ghorbani S, Goodarzi A (2018) In vitro and in vivo study of PCL/COLL wound dressing loaded with insulin-chitosan nanoparticles on cutaneous wound healing in rats model. Int J Biol Macromol 117:601–609
Tanha S, Rafiee-Tehrani M, Abdollahi M, Vakilian S, Esmaili Z, Naraghi ZS, Seyedjafari E, Javar HA (2017) G-CSF loaded nanofiber/nanoparticle composite coated with collagen promotes wound healing in vivo. J Biomed Mater Res A 105:2830–2842
Archana D, Dutta J, Dutta PK (2013) Evaluation of chitosan nano dressing for wound healing: characterization, in vitro and in vivo studies. Int J Biol Macromol 57:193–203
Feng P, Luo Y, Ke C, Qiu H, Wang W, Zhu Y, Hou R, Xu L, Wu S (2021) Chitosan-based functional materials for skin wound repair: mechanisms and applications. Front Bioeng Biotechnol 9:650598. https://doi.org/10.3389/fbioe.2021.65059
Chong C, Wang Y, Fathi A, Parungao R, Maitz PK, Li Z (2019) Skin wound repair: Results of a pre-clinical study to evaluate electropsun collagen-elastin-PCL scaffolds as dermal substitutes. Burns 45:1639–1648
Samadieh S, Dehnad A, Naghili B, Sadri M, Bazmani A (2019) Hemocompatibility assessment and drug release kinetics investigation from materials based on electrospun nanofibers. Nanomed Res J 4:10–15
Dey RK, Ray AR (2003) Synthesis, characterization, and blood compatibility of polyamidoamines copolymers. Biomaterials 24:2985–2993
Nazarov R, Jin HJ, Kaplan DL (2004) Porous 3-D scaffolds from regenerated silk fibroin. Biomacromol 5:718–726
Alhosseini SN, Moztarzadeh F, Mozafari M, Asgari S, Dodel M, Samadikuchaksaraei A, Kargozar S, Jalali N (2012) Synthesis and characterization of electrospun polyvinyl alcohol nanofibrous scaffolds modified by blending with chitosan for neural tissue engineering. Int J Nanomed 7:25–34
Naseri-Nosar M, Salehi M, Hojjati-Emami S (2017) Cellulose acetate/poly lactic acid coaxial wet-electrospun scaffold containing citalopram-loaded gelatin nanocarriers for neural tissue engineering applications. Int J Biol Macromol 103:701–708
Pezeshki-Modaress M, Zandi M, Rajabi S (2018) Tailoring the gelatin/chitosan electrospun scaffold for application in skin tissue engineering: an in vitro study. Prog Biomater 7:207–218
Autian J (1975) In: Kronenthal RL, Oser Z, Martin E (ed) Polymers in Medicine and Surgery, Polymer Science and Technology Series, Springer, New York
Li CW, Wang Q, Li J, Hu M, Shi SJ, Li ZW, Wu GL, Cui HH, Li YY, Zhang Q, Yu XH, Lu LC (2016) Silver nanoparticles/chitosan oligosaccharide/poly(vinyl alcohol) nanofiber promotes wound healing by activating TGFβ1/Smad signaling pathway. Int J Nanomed 11:373–386
Archana D, Singh BK, Dutta J, Dutta PK (2015) Chitosan-PVP-nano silver oxide wound dressing: in vitro and in vivo evaluation. Int J Biol Macromol 73:49–57
Memic A, Abudula T, Mohammed HS, Joshi Navare K, Colombani T (2019) Bencherif, S.A. Latest Progress in Electrospun Nanofibers for Wound Healing Applications. ACS Appl Bio Mater 2:952–969
Augustine R, Rehman SRU, Ahmed R et al (2020) Electrospun chitosan membranes containing bioactive and therapeutic agents for enhanced wound healing. Int J Biol Macromol 156:153–170
Fahimirad S, Ajalloueian F (2019) Naturally-derived electrospun wound dressings for target delivery of bio-active agents. Int J Pharm 566:307–328
Saghazadeh S, Rinoldi C, Schot M, Kashaf SS, Sharifi F, Jalilian J, Nuutila K, Giatsidis G, Mostafalu P, Derakhshandeh H, Yue K, Swieszkowski W, Memic A, Tamayol A, Khademhosseini A (2018) Drug delivery systems and materials for wound healing applications. Adv Drug Deliv Rev 127:138–166
Jiang H, Wang XB, Li CY, Li JS, Xu FJ, Mao C, Yang WT, Shen J (2011) Improvement of hemocompatibility of polycaprolactone film surfaces with zwitterionic polymer brushes. Langmuir 27:11575–11581
Bagheri M, Validi M, Gholipour A, Makvandi P, Sharifi E (2022) Chitosan nanofiber biocomposites for potential wound healing applications: Antioxidant activity with synergic antibacterial effect. Bioeng Transl Med. https://doi.org/10.1002/btm2.10254
Garcia Garcia CE, Bossard F, Rinaudo M (2021) Electrospun biomaterials from Chitosan blends applied as scaffold for tissue regeneration. Polymers (Basel). https://doi.org/10.3390/polym13071037
Farzamfar S, Salehi M, Tavangar SM, Verdi J, Mansouri K, Ai A, Malekshahi ZV, Ai J (2019) A novel polycaprolactone/carbon nanofiber composite as a conductive neural guidance channel: an in vitro and in vivo study. Prog Biomater 8:239–248
Fahimirad S, Abtahi H, Satei P, Ghaznavi-Rad E, Moslehi M, Ganji A (2021) Wound healing performance of PCL/chitosan based electrospun nanofiber electrosprayed with curcumin loaded chitosan nanoparticles. Carbohydr Polym. https://doi.org/10.1016/j.carbpol.2021.117640
Miguel SP, Ribeiro MP, Coutinho P, Correia IJ (2017) Electrospun polycaprolactone/aloe vera_chitosan nanofibrous asymmetric membranes aimed for wound healing applications. Polymers 9(5):183
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kaliaperumal, C., Thulasisingh, A. In-vitro and in-vivo assessment of Polycaprolactone-Chitosan-Pectin imbibed nanofiber potentials as a wound healing biomaterial. J Polym Res 30, 160 (2023). https://doi.org/10.1007/s10965-023-03545-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-023-03545-5