Abstract
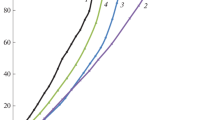
The kinetic study of free radical polymerization of styrene in the presence of n-pentane is a challenging and relevant topic because of its commercial and scientific value. Although this reaction has been carried out for decades, its kinetics are not fully understood. Thus, this polymerization is a current research topic mainly focused on the diffusive effects and ways to make more economical and efficient processes. This work presents a study focused on analyzing the kinetic effect of n-pentane addition during the bulk free-radical polymerization of styrene to contribute to the comprehension of phenomena which take place during this reaction. To reach this goal, we extended and implemented a model previously developed by one of the authors to perform the simulation and prediction of experimental data reported in the literature at different reaction conditions and multiple n-pentane amounts. Results obtained with the model allow us to explain the polymerization evolution in terms of the diffusive effects and the explicit theoretical description of the repetitive units that a terminal segment from a long radical involved in the termination stage can move (Usegm). That lets us identify the effects produced by the presence of n-pentane in the theoretical transition region from chemical to diffusive control in the termination reaction. This region is relevant due to its relationship with the increasing polymerization rate and the onset of the auto-acceleration effect. The comparison of the theoretical evolution of the weight-average molecular weight (Mw) against experimental data indicates that to enhance their correlation is necessary to consider not only the solvation effect but also the transfer effect produced by the presence of n-pentane during the polymerization.













Similar content being viewed by others
References
Marten B, Hicks A (2018) Expanded polystyrene life cycle analysis literature review: an analysis for different disposal scenarios. Sustain J Rec 11:29–35. https://doi.org/10.1089/sus.2017.0015
Ramli Sulong NH, Mustapa SAS, Abdul Rashid MK (2019) Application of expanded polystyrene (EPS) in buildings and constructions: a review. J Appl Polym Sci 136:47529. https://doi.org/10.1002/app.47529
Hajova H, Chmelar J, Nistor A, Gregor T, Kosek J (2013) Experimental study of sorption and diffusion of n-pentane in polystyrene. J Chem Eng Data 58:851–865. https://doi.org/10.1021/je300916f
Villalobos MA, Hamielec AE, Wood PE (1993) Bulk and suspension polymerization of styrene in the presence of n-pentane. An evaluation of monofunctional and bifunctional initiation. J Appl Polym Sci 50:327–343. https://doi.org/10.1002/app.1993.070500214
De Keer L, Van Steenberge PH, Reyniers MF, Marin GB, Hungenberg KD, Seda L, D’hooge DR (2017) A complete understanding of the reaction kinetics for the industrial production process of expandable polystyrene. AIChE J 63:2043–2059. https://doi.org/10.1002/aic.15587
Victoria-Valenzuela D, Herrera-Ordonez J, Luna-Barcenas G (2016) Toward a general methodology for modeling diffusive-controlled reactions in free radical polymerization. Macromol Theory Simul 25:28–44. https://doi.org/10.1002/mats.201500025
Victoria-Valenzuela D, Herrera-Ordonez J, Ricardo CLA, Estévez M (2018) Autoacceleration in bulk free-radical polymerization: effect of chain transfer. Macromol Chem Phys 219:1700434. https://doi.org/10.1002/macp.201700579
Vivaldo-Lima E, Saldívar-Guerra E (2013) Polymer reaction engineerin. Handbook of polymer synthesis, characterization, and processing. John Wiley & Sons, Incorporated, pp 253–255
Konstadinidis K, Achilias DS, Kiparissides C (1992) Development of a unified mathematical framework for modelling molecular and structural changes in free-radical homopolymerization reactions. Polymer 33:5019–5031. https://doi.org/10.1016/0032-3861(92)90053-Y
Saldívar-Guerra E, Infante-Martínez R, Vivaldo-Lima E, Flores-Tlacuahuac A (2010) Returning to basics: direct integration of the full molecular-weight distribution equations in addition polymerization. Macromol Theory Simul 19:151–157. https://doi.org/10.1002/mats.200900072
Tsuchida E, Tomono T, Sano H (1972) Solvent effects on the alternating copolymerization systems. Evaluation on equilibrium constants by NMR spectroscopy. Die Makromolekulare Chemie. Macromol Chem Phys 151:245–264. https://doi.org/10.1002/macp.1972.021510119
Vrentas JS, Duda JL, Ling HC (1984) Self-diffusion in polymer-solvent-solvent systems. J Polym Sci Polym Phys Ed 22:459–469. https://doi.org/10.1002/pol.1984.180220308
Arya RK, Thapliyal D, Sharma J, Verros GD (2021) Glassy polymers—Diffusion, sorption, ageing and applications. Coatings. https://doi.org/10.3390/coatings11091049
Pickup S, Blum FD (1989) Self-diffusion of toluene in polystyrene solutions. Macromolecules 22:3961–3968. https://doi.org/10.1021/ma00200a025
Hong SU (1995) Prediction of polymer/solvent diffusion behavior using free-volume theory. Ind Eng Chem Res 34:2536–2544. https://doi.org/10.1021/ie00046a040
Vrentas JS, Duda JL, Ling HC, Hou AC (1985) Free-volume theories for self-diffusion in polymer–solvent systems. II. Predictive capabilities. J Polym Sci: Polym Phys Ed 23:289–304. https://doi.org/10.1002/pol.1985.180230205
Achilias DS, Kiparissides C (1992) Development of a general mathematical framework for modeling diffusion-controlled free-radical polymerization reactions. Macromolecules 25:3739–3750. https://doi.org/10.1021/ma00040a021
Van Hook JP, Tobolsky AV (1958) The initiator efficiency of 2, 2′-azobisisobutyronitrile in the polymerization of styrene and methyl methacrylate, and dicumyl peroxide as a polymerization initiator. J Polym Sci 33:429–445. https://doi.org/10.1002/pol.1958.1203312640
Novakovic K, Martin EB, Morris AJ (2003) Modelling of the free radical polymerization of styrene with benzoyl peroxide as initiator. In: Computer Aided Chemical Engineering, Elsevier, pp 815–820
Bertin D, Boutevin B (1996) Controlled radical polymerization. Polym Bull 37:337–344. https://doi.org/10.1007/BF00318066
Soh SK, Sundberg DC (1982) Diffusion-controlled vinyl polymerization. I. The gel effect. J. Polym. Sci. Polym Chem Ed 20:1299–1313. https://doi.org/10.1002/pol.1982.170200513
Russell GT, Napper DH, Gilbert RG (1988) Initiator efficiencies in high-conversion bulk polymerizations. Macromolecules 21:2141–2148. https://doi.org/10.1021/ma00185a045
Gilbert RG (1995) In: Emulsion polymerization, a mechanistic approach. Academic Press, London, p 121
Zhu S, Hamielec AE (1989) Chain-length-dependent termination for free radical polymerization. Macromolecules 22:3093–3098. https://doi.org/10.1021/ma00197a033
Dhib R, Gao J, Penlidis A (2000) Simulation of free radical bulk/solution homopolymerization using mono-and bi-functional initiators. Polym React Eng 8:299–464. https://doi.org/10.1080/10543414.2000.10744557
Van Herk AM (2000) Pulsed initiation polymerization as a means of obtaining propagation rate coefficients in free-radical polymerizations. II Review up to 2000. Macromol Theory Simul 9:433–441. https://doi.org/10.1002/1521-3919(20001101)9:8%3c433::AID-MATS433%3e3.0.CO;2-I
Acknowledgements
Victoria-Valenzuela thanks the Consejo Nacional de Ciencia y Tecnología (CONACYT) for the postdoctoral fellowship corresponding to the call: ESTANCIAS POSDOCTORALES POR MÉXICO MODALIDAD 1. Into the Master program; in Science in Chemical Engineering (TecNM campus Cd. Madero). The authors also wish to thank the anonymous reviewers for their comments and suggestions and to Dr. Cabrero Martinez Omar A. for proofreading assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest. All authors contributed equally to this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Victoria-Valenzuela, D., Morales-Cepeda, A.B. Contributing to a better comprehension of: the styrene bulk free-radical polymerization with n-pentane added as a blowing agent for producing expandable polystyrene. J Polym Res 29, 392 (2022). https://doi.org/10.1007/s10965-022-03201-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-022-03201-4