Abstract
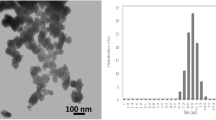
A semi interpenetrating polymer network (SIPN) of Aloe-vera-poly(acrylic acid) and its nano-composite were synthesized by free radical polymerisation method. Effect of various chemical properties on percentage swelling of nano-composites was investigated. Nanoparticles in SIPN was characterised by FTIR, Field emission scanning electron microscopy (FESEM), transmission electron microscopy (TEM), thermal gravimetric analysis (TGA) and electron dispersive spectroscopy (EDS). Synthesized nanocomposite was evaluated as an adsorbent for the adsorption of carcinogenic dye malachite green. Optimised condition for removal of malachite green dye from synthesized nanocomposite was pH 10, time 16 h, dye concentration 10 ppm and adsorbent dosage 0.05g. Maximum dye removal exhibited by the synthesized nano composite was 98.2%. Dye adsorption isotherm and kinetics were best explained through Freundlich adsorption isotherm and pseudo second order model, exhibiting its correlation coefficient (R2)0.99569 and 0.99271. Thus, synthesized nano-composite was utilised as an efficient device for dye sorption.









Similar content being viewed by others
Data availability
The whole data of the present manuscript is available; it will be provided if asked.
Change history
26 August 2022
A Correction to this paper has been published: https://doi.org/10.1007/s10965-022-03235-8
References
Biondi M, Borzacchiello A, Mayol L, Ambrosio L (2015) Nanoparticle-integrated hydrogels as multifunctional composite materials for biomedical applications. Gels 1:162–178
Henrique P, Camargo C, Satyanarayana G, Wypychol F (2009) Nano-composites: synthesis, structure, properties and new application opportunities. Mater Res 1:1516–1439
France K, Hoare T, Emily D (2017) Review of Hydrogels and Aerogels Containing Nanocellulose. Cranston Chemistry of Material 29:4609–4631
Naushad M, Mittal A, Rathore M, Gupta V (2015) Ion-exchange kinetic studies for Cd(II), Co(II), Cu(II), and Pb(II) metal ions over a composite cation exchanger. Desalin Water Treat 54:2883–2890
Ahmed DN, Naji LA, Faisal AAH, Al-Ansari N, Naushad M (2020) Waste foundry sand/MgFe-layered double hydroxides composite material for efficient removal of Congo red dye from aqueous solution. Sci Rep 10(1):1–12
Tetiana Tatarchuk DZ, Paliychuk N, Bitra RB, Shyichuk A, Naushad M, Mironyuk I (2019) Adsorptive removal of toxic Methylene Blue and Acid Orange 7 dyes from aqueous medium using cobalt-zinc ferrite nanoadsorbents. Desalin Water Treat 150:374–385
Adegoke Adesina K, Bello O (2015) Dye sequestration using agricultural wastes as adsorbents. Water Resour Ind 12:8–24
Gisi S, GiusyLofrano G, Mariangela Grass M, Notarnicola M (2016) Characteristics and adsorption capacities of low-cost sorbents for waste water treatment: Areview. Sustain Mater Technol 9:10–40
Sharma G, Dionysiou DD, Sharma S, Kumar A, Al-Muhtaseb AH, Naushad M, Stadler FJ (2019) Highly efficient Sr/Ce/activated carbon bimetallic nanocomposite for photoinduced degradation of rhodamine B. Catal Today 335
Sharma G, Kumar A, Sharma S, Naushad M, Dhiman P, Vo DVN, Stadler FJ (2020) Fe3O4/ZnO/Si3N4 nanocomposite based photocatalyst for the degradation of dyes from aqueous solution. Mater Lett 278:128359
Hu X, Liang R, Sun G (2018) Super-adsorbent hydrogel for removal of methylene bluedye from aqueous solution. J Mater Chem 6:17612–17624
Chowdhury A, Khan A, Kumari S, Hussain S (2019) Superadsorbent Ni-Co-S/SDS nanocomposites for ultrahigh removal of cationic, anionic organic dyes and toxic metal ions: kinetics, isotherm and adsorption mechanism. Sustain Chem Eng 74:4165–4176
Saruchi Kumar V (2019) Separation of crude oil from water using chitosan based hydrogel. Cellulose 26:6229–6239
Kaith BS, Saruchi Jindal R, Bhatti MS (2012) Screening and RSM optimization for synthesis of a gum tragacanth–acrylic acid based device for in situ controlled cetirizine dihydrochloride release. Soft Matter 8:2286–2293
Naushad M, Vasudevan S, Sharma G, Kumar A, Alothman ZA (2016) Adsorption kinetics, isotherms, and thermodynamic studies for Hg2+ adsorption from aqueous medium using alizarin red-S-loaded amberlite IRA-400 resin. Desalin Water Treat 57:18551–18559
Naushad M, ALOthman ZA (2015) Separation of toxic Pb2+ metal from aqueous solution using strongly acidic cation-exchange resin: analytical applications for the removal of metal ions from pharmaceutical formulation. Desalin Water Treat 53(2015):2158–2166
Sadrykiaa F, Arsalania N (2015) Preparation and characterization of hydrogel Nano-composites based on oxidized starch and incompletely condensed polyhedral oligomeric silsesquioxanes. Procedia Mater Sci 11:531–535
Saruchi Kumar V, Kaith BS (2016) Synthesis of hybrid ion exchanger for rhodamine B dye removal: equilibrium, kinetic and thermodynamic studies. I & EC Research 55(39):10492–10499
Mohamed MG, Ahmed FMELM, Meng TS, Samy MM, Kuo SW (2020) Multifunctional hypercrosslinked porous organic polymers based on tetraphenylethene and triphenylamine derivatives for high performance dye adsorption and supercapacitor. Polymers 12(10):2426–2443
Saruchi Kaith B, Jindal R, Kumar V, Bhatti M (2014) Optimal response surface design of Gum tragacanth- based poly[(acrylic acid)-coacrylamide] IPN hydrogel for the controlled release of the antihypertensive drug losartan potassium. RSC Advance 4:39822–39829
Mittal H, Mishra S, Mishra A, Kaith B, Jindal R, Kalia S (2013) Preparation of poly(acrylamide-co-acrylic acid)-grafted gum and its flocculation and biodegradation studies. Carbohydr Polym 98:397–404
Shen Y, Li W, Hu Z (2010) Preparation of calcium alginate sorbent supporting the BaSO4-APRB hybrid and application to clean dye waste. J Food Agric Environ 8:956–961
Kaith B, Sharma J, Sukriti Sethi S, Kaur T, Shanker U, Jassal V (2016) Fabrication of green device for efficient capture of toxic methylene blue from industrial effluent based on K2Zn3[Fe(CN)6]2·9H2O nanoparticles reinforced gum xanthan-psyllium hydrogel nano-composite. Journal of the Chinese Advanced Materials Society 4:249–268
Li J, Xie H, Chen L (2011) A sensitive hydrazine electrochemical sensor based on electrodeposition of gold nanoparticles on choline film modified glassy carbon electrode. Sensors and Actuators B Chem 153:239–245
UddinE Kim N, Kuila T, Lee S, Hui D, Lee J (2015) Preparation of reduced graphene oxide-NiFe2O4nano-composites for the electrocatalytic oxidation of hydrazine. Compos Part B Eng 79:649–659
Charurvedi S, Dave P (2012) Current microscopy contributions to advances in science and technology, microscopy in nanotechnology. 946–952
Brydson R, Brown A (2008) An investigation of the surface structure of nanoparticulate systems using analytical electron microscopes corrected for spherical aberration. Turning Points in Solid-State. RSC Publishing, Material and Surface Science. London, pp 778–791
Hadi A (2013) Dye removal from coloured textile wastewater using synthesized chitosan. Int J Sci Technol 2:2049–7318
Thakur P, Kumar V(2019), Kinetics and thermodynamic studies for removal of methylene blue dye by biosynthesize copper oxide nanoparticles and its antibacterial activity. J Environ Health Sci Eng 17:367–376. https://doi.org/10.1007/s40201-019-00354-1
Sethi S, Kaith BS, Saruchi Kumar V (2019) Fabrication and characterization of microwave assisted carboxymethyl cellulose-gelatin silver nanoparticles imbibed hydrogel: Its evaluation as dye degradation. React Funct Polym 142:134–146
Aly KI, Sayed MM, Mohamed MG, Kuo SW, Younis O (2020) A facile synthetic route and dual function of network luminescent porous polyester and copolyester containing porphyrin moiety for metal ions sensor and dyes adsorption. Microporous and Mesoporous Mater 298:110063
Kaur K, Jindal R (2019) Comparative study on the behaviour of Chitosan-Gelatin based hydrogel and nano-composite ion exchanger synthesized under microwave conditions towards photocatalytic removal of cationic dyes. Carbohydr Polym 207:398–410
Alizadeh N, Mahjoub M (2017) Removal of crystal violet dye from aqueous solution using surfactant modified NiFe2O4 as nanoadsorbent isotherms, thermodynamics and kinetics studies. J Nanoanalysis 4:8–19
Kumar R, Sharma R, Singh A (2018) Removal of organic dyes and metal ions by cross-linked graft copolymers of cellulose obtained from the agricultural residue. J Environ Chem Eng 6:6037–6048
Mittal H, Mishra SB (2014) Gum ghatti and Fe3O4 magnetic nanoparticles based nano-composites for the effective adsorption of rhodamine B. Carbohydr Polym 101:1255–1264
Saruchi Kumar V (2019) Adsorption kinetics and isotherms for the removal of rhodamine B dye and Pb+2 ions from aqueous solutions by a hybrid ion-exchanger. Arab J Chem 12:316–329
Saruchi Kaith BS, Kumar V, Jindal R (2016) Biodegradation study of enzymatically catalyzed interpenetrating polymer network: evaluation of agrochemical release and impact on soil fertility. Biotechnol Rep 9:74–81
Acknowledgement
One of the authors Vishal Rehani also acknowledges the support from Research, Innovative and Consultancy Department, Punjab Technical University, Kapurthala.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
No animal is utilized for carrying out this research work.
Consent to participate/consent to publish
Not applicable for this work.
Conflict of interest
There is no conflict of interest regarding this manuscript
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Saruchi, Kumar, V., Dhami, J.K. et al. Synthesis and characterization of Aloe-vera-poly(acrylic acid)-Cu-Ni-bionanocomposite: its evaluation as removal of carcinogenic dye malachite green. J Polym Res 29, 49 (2022). https://doi.org/10.1007/s10965-022-02898-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-022-02898-7