Abstract
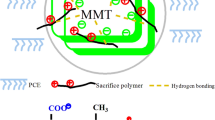
Polycarboxylate-based water reducing admixtures (PCE) are widely used in concrete technology as they have superior properties such as ease of molecular structure change, high dispersing and water reduction capacity. Besides these positive effects, PCEs show a high sensitivity to clay particles. Surface adsorption and intercalation of PCEs with clays reduce their dispersing and water reduction capacity. The action-performance of PCE depends on the morphological properties of clays and the chemical structure of PCEs. Approaches such as increasing PCE dosage, adding polyethylene-glycol (PEG)—grafted lignin, and using clay tolerant sacrificial agents were proposed to avoid the adverse effect of clays on the action performance of PCE. However, as these approaches are not economical, there is a need to synthesize modified PCEs. In this study, PCE-clay interaction and methods to increase the action-performance of PCE in systems containing clay were investigated in detail. It was reported that the addition of functional groups to PCEs or use of PCEs with short polyethylene oxide (PEO) side chain lengths limit intercalation. In addition, it was emphasized that the size of the side chain shape that the clays cannot be included in the interlayer region prevents intercalation and increases the action-performance of PCE.




















Similar content being viewed by others
Availability of data and material
All data, models, and code generated or used during the study appear in the submitted article.
References
Xu H, Sun S, Wei J, Yu Q, Shao Q, Lin C (2015) β-Cyclodextrin as pendant groups of a polycarboxylate superplasticizer for enhancing clay tolerance. Ind Eng Chem Res 54(37):9081–9088
Liu X, Guan J, Lai G, Zheng Y, Wang Z, Cui S, Lan M, Li H (2017) Novel designs of polycarboxylate superplasticizers for improving resistance in clay-contaminated concrete. J Ind Eng Chem 55:80–90. https://doi.org/10.1016/j.jiec.2017.06.031
Altun MG, Özen S, Mardani-Aghabaglou A (2021) Effect of Side Chain Length Change of Polycarboxylate-Ether–Based High-Range Water-Reducing Admixture on Properties of Cementitious Systems Containing Fly Ash. J Mater Civ Eng 33(4):04021015
Uchikawa H, Hanehara S, Sawaki D (1997) The role of steric repulsive force in the dispersion of cement particles in fresh paste prepared with organic admixture. Cem Concr Res 27(1):37–50. https://doi.org/10.1016/S0008-8846(96)00207-4
Li CZ, Feng NQ, Chen RJ (2005) Effects of polyethlene oxide chains on the performance of polycarboxylate-type water-reducers. Cem Concr Res 35(5):867–873. https://doi.org/10.1016/j.cemconres.2004.04.031
Dinari M, Roghani N (2021) Effect of triazine based silane coupling agent modified LDH on the thermal and mechanical properties of PVC based nanocomposites. J Polym Res 28(8):1–8
Ma Y, Shi C, Lei L, Sha S, Zhou B, Liu Y, Xiao Y (2020) Research progress on polycarboxylate based superplasticizers with tolerance to clays-A review. Constr Build Mater 255:119386. https://doi.org/10.1016/j.conbuildmat.2020.119386
Yamada K, Takahashi T, Hanehara S, Matsuhisa M (2000) Effects of the chemical structure on the properties of polycarboxylate-type superplasticizer. Cem Concr Res 30(2):197–207
Ran QP, Liu JP, MIU C (2010) Effect of the side chain length of comb-like copolymer superplasticizer on early hydration properties of concentrated cement suspensions. J Chin Ceram Soc 38(9):1718–1722
Özen S, Altun MG, Mardani-Aghabaglou A, Ramyar K (2021) Effect of main and side chain length change of polycarboxylate-ether-based water-reducing admixtures on the fresh state and mechanical properties of cementitious systems. Struct Concr 22:E607–E618
Lange A, Hirata T, Plank J (2014) Influence of the HLB value of polycarboxylate superplasticizers on the flow behavior of mortar and concrete. Cem Concr Res 60:45–50. https://doi.org/10.1016/j.cemconres.2014.02.011
Yu R, Spiesz PHJH, Brouwers HJH (2015) Development of an eco-friendly Ultra-High Performance Concrete (UHPC) with efficient cement and mineral admixtures uses. Cement Concr Compos 55:383–394
Pfeifer C, Moeser B, Stark J (2010) Hydration, phase and microstructure development of ultra-high performance concrete. ZKG Int 63(10):71–79
Lei L, Plank J (2012) A concept for a polycarboxylate superplasticizer possessing enhanced clay tolerance. Cem Concr Res 42(10):1299–1306. https://doi.org/10.1016/j.cemconres.2012.07.001
Tan H, Qi C, Ma B, Li X, Jian S (2015) Effect of polycarboxylate superplasticiser adsorption on fluidity of cement–clay system. Mater Res Innovations 19(sup5):S5-423. https://doi.org/10.1179/1432891714Z.0000000001124
Norvell JK, Stewart JG, Juenger MC, Fowler DW (2007) Influence of clays and clay-sized particles on concrete performance. J Mater Civ Eng 19(12):1053–1059
Magarotto R, Moratti F, Zeminian N (2006) Influence of sulfates content in cement on the performances of superplasticizers. Special Publication 239:215–230
Jeknavorian AA, Jardine L, Ou CC, Koyata H, Folliard K (2003) Interaction of superplasticizers with clay-bearing aggregates. Special Publication 217:143–160
Sakai E, Atarashi D, Daimon M (2006) Interaction between superplasticizers and clay minerals. In Proceedings of the 6th International Symposium on Cement & Concrete (Vol. 2, pp. 1560–1566)
Chan WWJ, Wu CML (2000) Durability of concrete with high cement replacement. Cem Concr Res 30(6):865–879
Beixing L, Mingkai Z, Jiliang W (2011) Effect of the methylene blue value of manufactured sand on performances of concrete. J Adv Concr Technol 9(2):127–132
Olanitori LM (2006) Mitigating the effect of clay content of sand on concrete strength. In 31st Conference on Our World in Concrete and Structures (pp. 15–17)
Li BX, Wang JL, Zhou MK (2009) C60 high performance concrete prepared from manufactured sand with a high content of microfines. In Key Engineering Materials (Vol. 405, pp. 204–211). Trans Tech Publications Ltd
Désiré TJ, Léopold M (2013) Impact of clay particles on concrete compressive strength. International Research Journal on Engineering 1(2):049–056
Fan Y, Zhang S, Kawashima S, Shah SP (2014) Influence of kaolinite clay on the chloride diffusion property of cement-based materials. Cement Concr Compos 45:117–124. https://doi.org/10.1016/j.cemconcomp.2013.09.021
Tugrul A, Hasdemir S, Yılmaz M (2015) The effect of feldspar, mica and clay minerals on compressive strength of mortar. In Engineering Geology for Society and Territory-Volume 5 (pp. 93–96). Springer, Cham
Batool R, Altaf F, Hameed MU, Abbas G, Kazmi SAR, Jacob K (2021) In situ chemical synthesis and characterization of PAN/clay nanocomposite for potential removal of Pb+ 2 ions from aqueous media. J Polym Res 28(8):1–15
Liu S, Mo X, Zhang C, Sun D, Mu C (2004) Swelling inhibition by polyglycols in montmorillonite dispersions. J Dispersion Sci Technol 25(1):63–66
Singh NB, Middendorf B, Gajbhiye NS, Kumar M (2012) Hydration of ternary blended cement in the presence of PC type superplasticizer. ZKG international (Deutsch-englische Ausgabe. 1995), 1:54–63. https://doi.org/10.1016/j.cemconres.2019.05.017
Burgos-Montes O, Palacios M, Rivilla P, Puertas F (2012) Compatibility between superplasticizer admixtures and cements with mineral additions. Constr Build Mater 31:300–309. https://doi.org/10.1016/j.conbuildmat.2011.12.092
Tang X, Zhao C, Yang Y, Dong F, Lu X (2020) Amphoteric polycarboxylate superplasticizers with enhanced clay tolerance: Preparation, performance and mechanism. Constr Build Mater 252:119052. https://doi.org/10.1016/j.conbuildmat.2020.119052
Tregger NA, Pakula ME, Shah SP (2010) Influence of clays on the rheology of cement pastes. Cem Concr Res 40(3):384–391. https://doi.org/10.1016/j.cemconres.2009.11.001
Atarashi D, Yamada K, Itoh A, Miyauchi M, Sakai E (2015) Interaction between montmorillonite and chemical admixture. J Adv Concr Technol 13(6):325–331. https://doi.org/10.3151/jact.13.325
Borralleras P, Segura I, Aranda MA, Aguado A (2019) Influence of experimental procedure on d-spacing measurement by XRD of montmorillonite clay pastes containing PCE-based superplasticizer. Cem Concr Res 116:266–272. https://doi.org/10.1016/j.cemconres.2018.11.015
Nehdi ML (2014) Clay in cement-based materials: Critical overview of state-of-the-art. Constr Build Mater 51:372–382. https://doi.org/10.1016/j.conbuildmat.2013.10.059
Yang Y, Ran QP, Mao YL, Zhang ZY, Liu JP (2012) Adsorption Behavior of Polycarboxylate Superplasticizers onto Montmorillonite. J Build Mater 4
You-guanga LI, Yuana LI, Yub WAN, Chenga DENG, Zhib WANG, Jue-shia QIAN (2012) Effects of clay on the dispersibility of cement paste mixed with polycarboxylate superplasticizer. Journal of Chongqing University
Lei L, Plank J (2014) A study on the impact of different clay minerals on the dispersing force of conventional and modified vinyl ether based polycarboxylate superplasticizers. Cem Concr Res 60:1–10. https://doi.org/10.1016/j.cemconres.2014.02.009
Lei L, Zhang Y, Li R (2021) Specific molecular design of polycarboxylate polymers exhibiting optimal compatibility with clay contaminants in concrete. Cem Concr Res 147:106504. https://doi.org/10.1016/j.cemconres.2021.106504
Köksal A, Abit Ö, & Karataş E (2013) Metilen mavisi değeri yüksek agregalar ve farklı özellikteki kimyasal katkılarla yapılan beton çalışmaları (In Turkish). Yapıchem.com.tr
Liu ZA, Zhou MK, Yao CK (2015) Relationship between methylene blue value of manufactured sand and mortar properties. In Key Engineering Materials (Vol. 629, pp. 612–617). Trans Tech Publications Ltd
Zheng T, Zheng D, Li X, Cai C, Lou H, Liu W, Qiu X (2017) Synthesis of quaternized lignin and its clay-tolerance properties in montmorillonite-containing cement paste. ACS Sustain Chem Eng 5(9):7743–7750
Lin X, Zhou M, Wang S, Lou H, Yang D, Qiu X (2014) Synthesis, structure, and dispersion property of a novel lignin-based polyoxyethylene ether from kraft lignin and poly (ethylene glycol). ACS ACS Sustain Chem Eng 2(7):1902–1909
Lange A, Plank J (2012) Study on the foaming behaviour of allyl ether-based polycarboxylate superplasticizers. Cem Concr Res 42(2):484–489. https://doi.org/10.1016/j.cemconres.2011.11.0172017
Schmid M, Plank J (2020) Dispersing performance of different kinds of polycarboxylate (PCE) superplasticizers in cement blended with a calcined clay. Constr Build Mater 258:119576. https://doi.org/10.1016/j.conbuildmat.2020.119576
Li R, Lei L, Sui T, Plank J (2021) Effectiveness of PCE superplasticizers in calcined clay blended cements. Cem Concr Res 141:106334. https://doi.org/10.1016/j.cemconres.2020.106334
Chen G, Lei J, Du Y, Chen X (2018) Synthesis of a novel polycarboxylate superplasticizer with carboxyl group as side chain terminal group to enhance its clay tolerance. J Wuhan Univ Technol Mater Sci Ed 33(1):226–232
Li Y, Zheng X, Wu K, Lu M (2016) Synthesis of amphiphilic polycarboxylate copolymer and its notable dispersion and adsorption characteristics onto cement and clay. Adv Cem Res 28(5):344–353
Anderson RL, Ratcliffe I, Greenwell HC, Williams PA, Cliffe S, Coveney PV (2010) Clay swelling—a challenge in the oilfield. Earth-Sci Rev 98(3–4):201–216. https://doi.org/10.1016/j.earscirev.2009.11.003
Kılınç K, Gök SG (2016) The Effect of Potassium Humate Based Chemical Admixtures on the Compressive Strength of Concrete Produced by Clay-Bearing Aggregates with High Methylene Blue Value. In 12th International Congress on Advances in Civil Engineering (ACE 2016), Bogazici University, Istanbul, Turkey
Vural P (2012) The effect of cement and zeolite (natural pozzolan) on engineering properties of clay soils with swell and dispersive characteristics. Master's thesis
Frank HS (1958) Covalency in the hydrogen bond and the properties of water and ice. Proceedings of the Royal Society of London. Series A J Math Phys Sci 247(1251):481–492
Pinnavaia TJ (1983) Intercalated clay catalysts. Science 220(4595):365–371
Tosun H (2011) The use of methylene blue test for predicting swell parameters of natural clay soils. Sci Res Essays 6(8):1780–1792
Van Olphen H (1977) An Introduction to Clay Colloid Chemistry: For Clay Technologists. Geologists, and Soil Scientists. 2
Miranda-Trevino JC, Coles CA (2003) Kaolinite properties, structure and influence of metal retention on pH. Appl Clay Sci 23(1–4):133–139. https://doi.org/10.1016/S0169-1317(03)00095-4
Rand B, Melton IE (1977) Particle interactions in aqueous kaolinite suspensions: I. Effect of pH and electrolyte upon the mode of particle interaction in homoionic sodium kaolinite suspensions. J Colloid Interface Sci 60(2):308–320. https://doi.org/10.1016/0021-9797(77)90290-9.
Grim RE (1962) Clay mineralogy: the clay mineral composition of soils and clays is providing an understanding of their properties. Science 135(3507):890–898
Tan H, Gu B, Guo Y, Ma B, Huang J, Ren J, Guo Y (2018) Improvement in compatibility of polycarboxylate superplasticizer with poor-quality aggregate containing montmorillonite by incorporating polymeric ferric sulfate. Constr Build Mater 162:566–575. https://doi.org/10.1016/j.conbuildmat.2017.11.166
Xi Y, Frost RL, He H (2007) Modification of the surfaces of Wyoming montmorillonite by the cationic surfactants alkyl trimethyl, dialkyl dimethyl, and trialkyl methyl ammonium bromides. J Colloid Interface Sci 305(1):150–158. https://doi.org/10.1016/j.jcis.2006.09.033
Seppälä A, Puhakka E, Olin M (2016) Effect of layer charge on the crystalline swelling of Na+, K+ and Ca2+ montmorillonites: DFT and molecular dynamics studies. Clay Miner 51(2):197–211
Ammann L (2003) Cation exchange and adsorption on clays and clay minerals (Doctoral dissertation)
Odom IE (1984) Smectite clay minerals: properties and uses. Philosophical Transactions of the Royal Society of London. Series A Math Phys Eng Sci 198311(1517):391–409
Mandalia T, Bergaya F (2006) Organo clay mineral–melted polyolefin nanocomposites effect of surfactant/CEC ratio. J Phys Chem Solids 67(4):836–845. https://doi.org/10.1016/j.jpcs.2005.12.007
Wang Z, Kao Y, Wang L et al (2015) Effect and mechanism of single mineral clay on dispersibility of polycarboxylate superplasticizer. J Build Mater 18:879–887
Ait-Akbour R, Boustingorry P, Leroux F, Leising F, Taviot-Guého C (2015) Adsorption of PolyCarboxylate Poly (ethylene glycol) (PCP) esters on Montmorillonite (Mmt): Effect of exchangeable cations (Na+, Mg2+ and Ca2+) and PCP molecular structure. J Colloid Interface Sci 437:227–234. https://doi.org/10.1016/j.jcis.2014.09.027
Ali H, Ismail AM (2021) Developing montmorillonite/PVDF/PEO microporous membranes for removal of malachite green: adsorption, isotherms, and kinetics. J Polym Res 28(11):1–17
Escamilla-Roa E, Nieto F, Sainz-Díaz CI (2016) Stability of the hydronium cation in the structure of illite. Clays Clay Miner 64(4):413–424
Tan H, Gu B, Ma B, Li X, Lin C, Li X (2016) Mechanism of intercalation of polycarboxylate superplasticizer into montmorillonite. Appl Clay Sci 129:40–46. https://doi.org/10.1016/j.clay.2016.04.020
Suter JL, Coveney PV (2009) Computer simulation study of the materials properties of intercalated and exfoliated poly (ethylene) glycol clay nanocomposites. Soft Matter 5(11):2239–2251
Lv S, Ju H, Qiu C, Ma Y, Zhou Q (2013) Effects of connection mode between carboxyl groups and main chains on polycarboxylate superplasticizer properties. J Appl Polym Sci 128(6):3925–3932
Plank J, Sakai E, Miao CW, Yu C, Hong JX (2015) Chemical admixtures—Chemistry, applications and their impact on concrete microstructure and durability. Cem Concr Res 78:81–99. https://doi.org/10.1016/j.cemconres.2015.05.016
Tan H, Li X, Liu M, Ma B, Gu B, Li X (2016) Tolerance of clay minerals by cement: effect of side-chain density in polyethylene oxide (PEO) superplasticizer additives. Clays Clay Miner 64(6):732–742. https://doi.org/10.1346/CCMN.2016.064037
Svensson PD, Hansen S (2010) Intercalation of smectite with liquid ethylene glycol—Resolved in time and space by synchrotron X-ray diffraction. Appl Clay Sci 48(3):358–367. https://doi.org/10.1016/j.clay.2010.01.006
Ng S, Plank J (2012) Interaction mechanisms between Na montmorillonite clay and MPEG-based polycarboxylate superplasticizers. Cem Concr Res 42(6):847–854. https://doi.org/10.1016/j.cemconres.2012.03.005
Wang Y, Xu C, Li H (2019) Property and Degradation Characteristics of Concrete Prepared with Aggregate Contained Montmorillonite. J Wuhan Univ Technol Mater Sci Ed 34(1):127–131
Liu J, Ran Q, Miao C, Zhou D (2011) Synthesis and characterization of comb-like copolymer dispersant with methoxy poly (ethylene oxide) side chains. Polym Plast Technol Eng 50(1):59–66
Zou F, Tan H, Guo Y, Ma B, He X, Zhou Y (2017) Effect of sodium gluconate on dispersion of polycarboxylate superplasticizer with different grafting density in side chain. J Ind Eng Chem 55:91–100. https://doi.org/10.1016/j.jiec.2017.06.032
Schmidt W, Brouwers HJH, Kühne HC, Meng B (2013) The working mechanism of starch and diutan gum in cementitious and limestone dispersions in presence of polycarboxylate ether superplasticizers. Appl Rheol 23(5)
Lange A, Plank J (2016) Contribution of non-adsorbing polymers to cement dispersion. Cem Concr Res 79:131–136
Wang Q, Li SY, Pan S, Guo ZW (2018) Synthesis and properties of a silane and copolymer-modified graphene oxide for use as a water-reducing agent in cement pastes. New Carbon Mater 33(2):131–139. https://doi.org/10.1016/S1872-5805(18)60330-0
Fan W, Stoffelbach F, Rieger J, Regnaud L, Vichot A, Bresson B, Lequeux N (2012) A new class of organosilane-modified polycarboxylate superplasticizers with low sulfate sensitivity. Cem Concr Res 42(1):166–172. https://doi.org/10.1016/j.cemconres.2011.09.006
Stecher J, Plank J (2016) Phosphated comb polymers-A new generation of highly effective superplasticizers. In 8th International RILEM Symposium on Self-Compacting Concrete SCC2016, Washington DC, USA
Werani M, Lei L (2021) Influence of side chain length of MPEG–based polycarboxylate superplasticizers on their resistance towards intercalation into clay structures. Constr Build Mater 281:122621. https://doi.org/10.1016/j.conbuildmat.2021.122621
Sun C, Zhou H, Li X, Wang, S, Xing J (2015) The clay-tolerance of amide-modified polycarboxylate superplasticizers and its performance with clay-bearing aggregates. In MEBE-International Conference on Materials, Environment and Biological Engineering (pp. 237–241)
Borralleras P, Segura I, Aranda MA, Aguado A (2020) Absorption conformations in the intercalation process of polycarboxylate ether based superplasticizers into montmorillonite clay. Constr Build Mater 236:116657. https://doi.org/10.1016/j.conbuildmat.2019.08.038
Xing G, Wang W, Fang G (2017) Cement dispersion performance of superplasticisers in the presence of clay and interaction between superplasticisers and clay. Adv Cem Res 29(5):194–205
Xing G, Wang W, Xu J (2016) Grafting tertiary amine groups into the molecular structures of polycarboxylate superplasticizers lowers their clay sensitivity. RSC Adv 6(108):106921–106927
Li Y, Zeng L, Zhou Y, Wang T, Zhang Y (2014) Preparation and characterization of montmorillonite intercalation compounds with quaternary ammonium surfactant: adsorption effect of zearalenone. J Nanomater 2014
Sheng-hua L, CAO Q, LI D, MA YJ, Qing-qiang L (2013) Preparation and Properties of a New Type of Polycarboxylate Superplasticizer Containing Benzene Ring. Fine Chem
Chen S, Zhang S, Zhang, S et al (2019) Design and performance test of snowflake antifouling polycarboxylate water reducer. J Build Mater 22:54–59
Zheng T, Zheng D, Qiu X, Yang D, Fan L, Zheng J (2019) A novel branched claw-shape lignin-based polycarboxylate superplasticizer: Preparation, performance and mechanism. Cem Concr Res 119:89–101. https://doi.org/10.1016/j.cemconres.2019.03.007
Felekoğlu B, Sarıkahya H (2008) Effect of chemical structure of polycarboxylate-based superplasticizers on workability retention of self-compacting concrete. Constr Build Mater 22(9):1972–1980. https://doi.org/10.1016/j.conbuildmat.2007.07.005
Konan KL, Peyratout C, Cerbelaud M, Smith A, Bonnet JP, Jacquet A (2008) Influence of two dispersants on the rheological behavior of kaolin and illite in concentrated calcium hydroxide dispersions. Appl Clay Sci 42(1–2):252–257. https://doi.org/10.1016/j.clay.2008.01.001
Plank J, Pöllmann K, Zouaoui N, Andres PR, Schaefer C (2008) Synthesis and performance of methacrylic ester based polycarboxylate superplasticizers possessing hydroxy terminated poly (ethylene glycol) side chains. Cem Concr Res 38(10):1210–1216. https://doi.org/10.1016/j.cemconres.2008.01.007
Sun C, Zheng GJ, Bi Y, Zhou H (2014) Synthesis for an Amide-ester Type Polycarboxylate Superplasticizer and its application in concrete. In Adv Mater Res 989:233–237. Trans Tech Publications Ltd
Zhang R, Yang L, Qin N, Tu R, Zhou J, Ye Z (2015) The swelled clay shrinkage property and inhibition of microcosmic migration in pores of quaternary ammonium salt cationic polymer. Polym Adv Technol 26(12):1544–1550
Hirata T, Branicio P, Ye J, Zheng J, Tomike Y, Lange A, Sullivan M (2017) Atomistic dynamics simulation to solve conformation of model PCE superplasticisers in water and cement pore solution. Adv Cem Res 29(10):418–428
Chen G, Lei J, Du Y, Du X, Chen XA (2018) polycarboxylate as a superplasticizer for montmorillonite clay in cement: Adsorption and tolerance studies. Arab J Chem 11(6):747–755. https://doi.org/10.1016/j.arabjc.2017.12.027
Qu H, Fu CE, Yang W, Yang Z, Zhang L (2018) Preparation, application and water reducing mechanism of a novel fluorescent superplasticizer with improved flow retaining ability and clay tolerance. J Disp Sci Tech 39(12):1829–1839
Yoshioka K, Tazawa EI, Kawai K, Enohata T (2002) Adsorption characteristics of superplasticizers on cement component minerals. Cem Concr Res 32(10):1507–1513
Zhang J, Liao B, Meng Y, Li S, Lin X, Huang J, Pang H (2020) Synthesis and properties of a novel amphoteric polycarboxylate superplasticizer. J Dispersion Sci Technol 41(4):628–637
Ren J, Luo S, Shi S, Tan H, Wang X, Liu M, Li X (2021) Synthesis and optimization of a montmorillonite-tolerant zwitterionic polycarboxylate superplasticizer via Box-Behnken design. Clay Miner 1–9
Liu X, Wang ZM, Wu H, Li HQ (2013) Effects of clay on flow properties of polycarboxylate superplasticizer and its control measure. In Advanced Materials Research (Vol. 690, pp. 682–685). Trans Tech Publications Ltd
Acknowledgements
The first author would like to acknowledge the scholarship provided by TUBITAK under Grant No. 219M425 during her Ph.D. study.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Şahin, H.G., Biricik, Ö. & Mardani-Aghabaglou, A. Polycarboxylate-based water reducing admixture – clay compatibility; literature review. J Polym Res 29, 33 (2022). https://doi.org/10.1007/s10965-021-02884-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-021-02884-5