Abstract
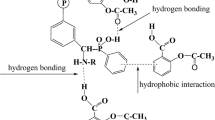
In the present study, poly(vinyl benzyl chloride) (PVBC) was prepared by the radical polymerization method. Then, PVBC was reacted with dibutyl amine to obtain tertiary amine-modified poly(styrene) based polymer (PVBC-Dibutyl amine) and the resulting polymer was characterized. Surface properties of the polymer were investigated by inverse gas chromatography method at infinite dilution region. For this purpose, the retention diagrams of carefully selected polar and non-polar solvents (probes) on PVBC-Dibutyl amine were obtained over a temperature range from 30 to 50 \(^\circ{\rm C}\). By using the diagrams, the dispersive component of the surface free energy of the polymer surface was calculated for the studied temperatures by two different methods. The specific enthalpy of adsorption and the specific component of the adsorption free energy of solvents on PVBC-Dibutyl amine which are adsorption thermodynamic parameters were determined for each solvent used. Using the adsorption data, Lewis acid and Lewis base parameters of PVBC-Dibutyl amine surface were calculated. The obtained parameters reflected that surface of PVBC-Dibutyl amine exhibited a basic behavior. Considering the values in the literature, the effect of dibutyl amine moieties in the structure of poly(styrene) based polymer PVBC-Dibutyl amine on the dispersive surface free energy and acid–base parameters of poly(styrene) was discussed.







Similar content being viewed by others
References
Voelkel A, Andrzejewska E, Maga R, Andrzejewski M (1996) Examination of surfaces of solid polymers by inverse gas chromatography: 1. Dispersive properties. Polymer 37:455–462
Zou QC, Zhang SL, Qq T, Wang SM, Wu LM (2006) Surface characterization of poly(methyl methacrylate-co-n-butyl acrylate-co-cyclopentylstyryl-polyhedral oligomeric silsesquioxane) by inverse gas chromatography. J Chromatogr A 1110:140–145
Ugraskan V, Isık B, Yazıcı O, Cakar F (2020) Thermodynamic characterization of sodium alginate by inverse gas chromatography. J Chem Eng Data 65:1795–1801
Topaloglu Yazıcı D, Askın A, Butun V (2006) Surface characteristics of 2-(diethylamino) ethyl methacrylate–2-(dimethylamino) ethyl methacrylate diblock copolymer determined by inverse gas chromatography. Surf Interface Anal 38:561–564
Smidsord O, Guillet JE (1969) Study of polymer solute interactions by gas chromatography. Macromol 2:272–277
Mohammadi-Jam S, Waters KE (2014) Inverse gas chromatography applications: A review. Adv Colloid Interface Sci 212:21–44
Adiguzel AC, Korkmaz B, Cakar F, Cankurtaran O, Hepuzer Gursel Y, Senkal BF, Karaman F (2020) Thermodynamics of a chalcone-based polymer by inverse gas chromatography method. Sigma J Eng Nat Sci 38:581–588
Ansari DM, Price GJ (2004a) Chromatographic estimation of filler surface energies and correlation with photodegradation of kaolin filled polyethylene. Polymer 45:1823–1831
Cava D, Gavara R, Lagaron J, Voelkel A (2007) Surface characterization of poly (lactic acid) and polycaprolactone by inverse gas chromatography. J Chromatogr A 1148:86–91
Santos José MRCA, Guthrie TJ (2015) Lewis acid/base character and crystallisation properties of poly(butylene terephthalate). J Chromatogr A 1379:92–99
Reddy AS, Rani PR, Reddy KS (2013) Lewis acid–base properties of cellulose acetate phthalate polycaprolactonediol blend by inverse gas chromatography. Polym Eng Sci 53:1780–1785
Adiguzel AC, Cakar F, Senkal BF, Cankurtaran O, Gursel Hepuzer Y, Karaman F (2019) Determination of glass transition temperature and surface properties of novel chalcone modified poly(styrene) based polymer. Therm Sci 23:193–202
Yazici O, Karaman F (2015) Determination of surface energies and acidity-basicity numbers of protonated and deprotonated forms of poly(sulfonic acid diphenyl aniline) by inverse gas chromatography. Polym Eng Sci 55:1246–1254
Papirer E, Brendle E, Balard H, Vergelati C (2000) Inverse gas chromatography investigation of the surface properties of cellulose. J Adhes Sci Technol 14:321–337
Chehimi M, Lascelles S, Armes P (1995) Characterization of surface thermodynamic properties of p-toluene sulfonate-doped polypyrrole by inverse gas chromatography. Chromatographia 41:671–677
Voelkel A (2004) Inverse gas chromatography in characterization of surface. Chemometr Intell Lab Syst 72:205–207
Mittal KL (2000) Acid-base interactions: relevance to adhesion science and technology 2. Taylor Francis Ltd., Boston
Minagawa M, Kanoh H, Tanno S, Nishimoto Y (2003) Glass transition temperature Tg of free radically prepared polyacrylonitrile by inverse gas chromatography 1. A study on Tg of atactic monodisperse polystyrenes. Macromol Chem Phys 203:2475–2480
Murakami Y, Inui T, Takegami Y (1983) Dispersion states of porous inorganic materials in polymer blends observed by inverse gas chromatography. Polymer 24:1596–1600
Ghaemy M, Hadjmohammadi M, Tabaraki R (2000) Study of crystallinity of high-density polyethylene by inverse gas chromatography. Iran Polym J 9:117–124
Ansari DM, Price GJ (2004b) Correlation of mechanical properties of clay filled polyamide mouldings with chromatographically measured surface energies. Polymer 45:3663–3670
Abel M-L, Chehimi MM, Fricker F, Delamar M, Brown AM, Watts JF (2002) Adsorption of poly (methyl methacrylate) and poly (vinyl chloride) blends onto polypyrrole: study by X-ray photoelectron spectroscopy, time-of-flight static secondary ion mass spectroscopy, and inverse gas chromatography. J Chromatogr A 969:273–285
Murakami Y, Enoki R, Ogoma Y, Kondo Y (1998) Studies on interaction between silica gel and polymer blend by inverse gas chromatography. Polym J 30:520–525
Onjia A, Milonjić S, Jovanović N, Jovanović S (2000) An inverse gas chromatography study of macroporous copolymers based on methyl and glycidyl methacrylate. React Funct Polym 43:269–277
Mekki A, Mettai B, Ihdene Z, Mahmoud R, Mekhalif Z (2013) Inverse gas chromatography characterization of polyaniline complexes: application to volatile organic compounds sensing. Iran Polym J 22:677–687
Rjiba N, Nardin M, Drean JY, Frydrych R (2009) Comparison of surfaces properties of different types of cotton fibers by inverse gas chromatography. J Polym Res 17:25–32
Clarke JT, Hammerschlag AH (1957) Mono (chloromethyl) styrene, its derivatives, and ion-exchange resins from polymers of aminated compound US2780604
Cyganowskia P, Cierlik A, Leśniewicz A, Pohl P, Jermakowicz-Bartkowiak D (2019) Separation of Re(VII) from Mo(VI) by anion exchange resins synthesized using microwave heat. Hydrometall 185:12–22
Gong MS, Lee CW (2003) Humidity-sensitive properties of gel polyelectrolyte based on cross-linked copolymers containing both ammonium salt and amine function. Mater Chem Phys 77:719–725
Monthéard JP, Jegat C, Camps M (2007) Vinylbenzylchloride (chloromethylstyrene) polymers and copolymers. Recent reactions and applications. J Macromol Sci Part C Polym Rev 39:135–174
Zhang X, Wang P, Zhu P, Ye C, Xi F (2000) Second order non-linear optical materials based on poly(p-chloromethyl styrene). Macromol Chem Phys 201:1853–1857
Noel C, Ching KC, Large M, Reyx D, Kajzar F (1997) Synthesis and characterization of polymers containing 4-cyanobiphenyl-based side groups for nonlinear optical applications, 3.† Poly(p-chloromethylstyrene) derivatives. Macromol Chem Phys 198:1665–1678
Yamashita K, KimuraY SH, Tsuda K (1991) Esterolysis of active esters by soluble thiol polymer. J Polym Sci Part A Polym Chem 29(5):777–779
Oughlis S, Lessim S, Changotade S, Bollotte F, Poirier F, Helary G, Lataillade JJ, Migonney V, Lutomski D (2011) Development of proteomic tools to study protein adsorption on a biomaterial, titanium grafted with poly(sodium styrene sulfonate). J Chromatogr B 879:3681–3687
Tabrizi MHN, Davaran S, Entezami AA (1996) Synthesis of diclofenac polymeric prodrugs and their hydrolysis reactivity. Iran Polym J 5:243–249
Dan M, Su Y, Xiao X, Li S, Zhang W (2013) A new family of thermo-responsive polymers based on poly[n-(4-vinylbenzyl)-n, n-dialkylamine]. Macromol 46:3137–3146
Papadopoulou SK, Dritsas G, Karapanagiotis I, Zuburtikudis I, Panayiotou C (2010) Surface characterization of poly (2,2,3,3,3-pentafluoropropyl methacrylate) by inverse gas chromatography and contact angle measurements. Eur Polym J 46:202–208
Conder JR, Young CL (1979) Physicochemical measurement by gas chromatography. Wiley, New York
Kiselev AV (1965) Non-specific and specific interactions of molecules of different electronic structures with solid surfaces. Discuss Faraday Soc 40:205–218
Dorris GM, Gray DG (1980) Adsorption of n-alkanes at zero surface coverage on cellulose paper and wood fibers. J Colloid Interface Sci 77:353–362
Schultz J, Lavielle L, Martin C (1987) The role of the interface in carbon fibre epoxy composites. J Adhes 23:45–60
Mukhopadhyay P, Schreiber H (1995) Aspects of acid–base interactions and use of inverse gas chromatography. Colloid Surf A Physicochem Eng Asp 100:47–71
Kamdem DP, Bose SK, Luner P (1993) Inverse gas chromatography characterization of birch wood meal. Langmuir 9:3039–3044
Riddle FL, Fowkes FM (1990) Spectral shifts in acid-base chemistry 1. van der Waals contributions to acceptor numbers. J Am Chem Soc 112:3259–3264
Santos JMRCA, Guthrie JT (2005a) Analysis of interactions in multicomponent polymeric systems: the key-role of inverse gas chromatography. Mater Sci Eng R Rep 50:79–107
Santos JMRCA, Guthrie JT (2005b) Study of a core-shell type impact modifier by inverse gas chromatography. J Chromatogr A 1070:147–154
Korkmaz B, Canlı NY, Ozdemir ZG, Okutan M, Hepuzer Gursel Y, Sarac A, Senkal BF (2016) Synthesis and electrical properties of hydrogen bonded liquid crystal polymer. J Mol Liq 219:1030–1035
Sohn S, Yang S (2003) On the work of adhesion and peel strength between pressure sensitive adhesives and the polymeric films used in LCD devices. J Adhes Sci Technol 17:903–915
Askın A, Etoz SS, Yazıcı DT, Tumsek F (2011) Examination of polystyrene by inverse GC: Part 1. below glass transition temperature. Chromatographia 73:109–115
Acknowledgment
This research has been supported by Yildiz Technical University Scientific Research Projects Coordination Department. Project Number: FDK-2018-3502
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Adıgüzel, A., Korkmaz, B., Çakar, F. et al. Investigation of the surface properties of dibutyl amine modified poly(styrene) based polymer by inverse gas chromatography method. J Polym Res 28, 83 (2021). https://doi.org/10.1007/s10965-021-02449-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-021-02449-6