Abstract
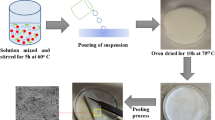
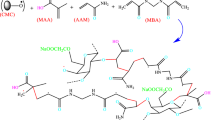
Reusable and eco-friendly poly(vinyl alcohol)/chitin/nanocellulose based biopolymer composite films were synthesized and characterized. Maleic acid (MA) was used as a crosslinker of the biopolymer composite film. The effect of constituent materials of the biopolymer composite film and the pH on removal of methylene blue (MB) dye from aqueous testing solution were studied by batch adsorption studies. The successful deposition of MB dye onto the adsorbent (biopolymer composite film) was confirmed using Fourier transform infrared spectroscopy (FTIR) analysis. Adsorption isotherm studies were fitted to the Freundlich model with the maximum adsorption capacity amounted to 467.5 mg/g. The adsorption kinetics were in conformance to the pseudo-second order model (R2 = 0.9924–0.9987). The point zero charge (pHpzc) of the adsorbents were investigated by pH drift method where the MA cross-linked adsorbents showed pHpzc values in the range of 8.05–8.55. Moreover, the best adsorption performance was observed in sample PVA/CT10/NCC/MA30, with calculated maximum adsorption capacity amounted to 467.5 mg/g. Thermodynamic studies showed that the adsorption were spontaneous, exothermic and less-ordered reactions. High adsorption reusability was determined for PVA/CT10/NCC/MA30 composite, with adsorption percentage of 83.67 ± 1.08% at the fifth cycle. All these positive results implied the potential application of PVA/Chitin/NCC composites for the MB dye’s adsorption from aqueous testing solution.







Similar content being viewed by others
References
Kaloo MA, Bhat BA, Sheergojri GA, Seh TI (2018) Elimination of dyes from waste water via adsorption materials. Mat Sci Res India 15(2):141–144. https://doi.org/10.13005/msri/150205
Rashed, MN (2013) Adsorption technique for the removal of organic pollutants from water and wastewater. In: Rashed MN (ed) Organic pollutants - monitoring, risk and treatment. InTech, Croatia, pp 167–171. doi:42059
Singh N, Nagpal G et al (2018) Water purification by using adsorbents: A review. Environ Technol Innov 11:187–240. https://doi.org/10.1016/j.eti.2018.05.006
Umoren S, Etim U et al (2013) Adsorption of methylene blue from industrial effluent using poly (vinyl alcohol). JMES 4(1):75–86
Akharame MO, Fatoki OS et al (2018) Polymeric nanocomposites (pncs) for wastewater remediation: An overview. Polym Plast Technol Eng 57(17):1801–1827. https://doi.org/10.1080/03602559.2018.1434666
Dhananasekaran S, Palanivel R et al (2016) Adsorption of methylene blue, bromophenol blue, and coomassie brilliant blue by α-chitin nanoparticles. J Adv Res 7(1):113–124. https://doi.org/10.1016/j.jare.2015.03.003
Li F, Wu X et al (2009) Adsorption and desorption mechanisms of methylene blue removal with iron-oxide coated porous ceramic filter. JWARP 1(1):35. https://doi.org/10.4236/jwarp.2009.11006
Olorundare O, Msagati T et al (2015) Polyurethane composite adsorbent using solid phase extraction method for preconcentration of metal ion from aqueous solution. Int J Environ Sci Technol 12(7):2389–2400. https://doi.org/10.1007/s13762-014-0645-5
Li L, Wang Z et al (2015) Preparation of polyvinyl alcohol/chitosan hydrogel compounded with graphene oxide to enhance the adsorption properties for cu (ii) in aqueous solution. J Polym Res 22(8):150
Moulay S (2015) Poly (vinyl alcohol) functionalizations and applications. Polym Plast Technol Eng 54(12):1289–1319. https://doi.org/10.1080/03602559.2015.1021487
Baheri B, Ghahremani R et al (2016) Dye removal using 4a-zeolite/polyvinyl alcohol mixed matrix membrane adsorbents: Preparation, characterization, adsorption, kinetics, and thermodynamics. Res Chem Intermed 42(6):5309–5328. https://doi.org/10.1007/s11164-015-2362-1
Rashidzadeh A, Olad A (2013) Novel polyaniline/poly (vinyl alcohol)/clinoptilolite nanocomposite: Dye removal, kinetic, and isotherm studies. Desalin Water Treat 51(37–39):7057–7066. https://doi.org/10.1080/19443994.2013.766904
Yang L, Li Y et al (2011) Preparation of novel spherical pva/atp composites with macroreticular structure and their adsorption behavior for methylene blue and lead in aqueous solution. Chem Eng J 173(2):446–455. https://doi.org/10.1016/j.cej.2011.08.003
Kamal H (2014) Removal of methylene blue from aqueous solutions using composite hydrogel prepared by gamma irradiation. J Am Sci 10(4):125–133
Yang X, Li Y et al (2016) Adsorption of methylene blue from aqueous solutions by polyvinyl alcohol/graphene oxide composites. J Nanosci Nanotechnol 16(2):1775–1782. https://doi.org/10.1166/jnn.2016.107081775
Casey LS, Wilson LD (2015) Investigation of chitosan-pva composite films and their adsorption properties. GEP 3(2):78–84. https://doi.org/10.4236/gep.2015.32013
Ghemati D, Aliouche D (2014) Dye adsorption behavior of polyvinyl alcohol/glutaraldehyde/β-cyclodextrin polymer membranes. J Appl Spectrosc 81(2):257–263. https://doi.org/10.1007/s10812-014-9919-4
Filipkowska U, Rodziewicz J (2009) Effectiveness of dye rb5 adsorption onto chitin and chitosan under static and dynamic conditions. PCACD 14:33–40
Lesbani A, Turnip EV et al (2015) Study adsorption desorption of manganese (ii) using impregnated chitin-cellulose as adsorbent. IJSER 8(2):104–108. https://doi.org/10.12777/ijse.8.2.104-108
Djelad A, Morsli A et al (2016) Sorption of cu (ii) ions on chitosan-zeolite x composites: Impact of gelling and drying conditions. Molecules 21(1):109. https://doi.org/10.3390/molecules21010109
Mok CK, Ching YC et al (2017) Poly(vinyl alcohol)-α-chitin composites reinforced by oil palm empty fruit bunch fiber-derived nanocellulose. Inter. J. Polym. Analy. and Characterization, Volume 22(4). doi.org/10.1080/1023666X.2017.1288345
Mok CF, Ching YC et al (2020) Preparation and characterization study on maleic acid cross‐linked poly (vinyl alcohol)/chitin/nanocellulose composites. J Appl Polym Sci:49044
Thennakoon MSUG, Ching YC et al (2017) Preparation and characterization of nanocellulose reinforced semi-interpenetrating polymer network of chitosan hydrogel. Cellulose 24:2215–2228
Tamura H, Nagahama H et al (2006) Preparation of chitin hydrogel under mild conditions. Cellulose 13(4):357–364. https://doi.org/10.1007/s10570-006-9058-z
Gatabi MP, Moghaddam HM et al (2016) Point of zero charge of maghemite decorated multiwalled carbon nanotubes fabricated by chemical precipitation method. J Mol Liq 216:117–125. https://doi.org/10.1016/j.molliq.2015.12.087
Ayawei N, Ebelegi AN et al (2017) Modelling and interpretation of adsorption isotherms. J Chem 2017
Faust SD, Aly OM (2013) Adsorption processes for water treatment. Elsevier
Agarwal S, Sadegh H et al (2016) Efficient removal of toxic bromothymol blue and methylene blue from wastewater by polyvinyl alcohol. J Mol Liq 218:191–197. https://doi.org/10.1016/j.molliq.2016.02.060
Ovchinnikov OV, Evtukhova AV et al (2016) Manifestation of intermolecular interactions in ftir spectra of methylene blue molecules. Vib Spectrosc 86:181–189. https://doi.org/10.1016/j.vibspec.2016.06.016
Zayed MF, Eisa WH et al (2016) Removal of methylene blue using phoenix dactylifera/pva composite; an eco-friendly adsorbent. Desalin Water Treat 57(40):18861–18867. https://doi.org/10.1080/19443994.2015.1094425
Hussin ZM, Talib N et al (2015) Methylene blue adsorption onto naoh modified durian leaf powder: Isotherm and kinetic studies. Am J Environ Engineer 5(3A):38–43. https://doi.org/10.5923/c.ajee.201501.07
El Feky A, Shalaby M et al (2010) Adsorption of some surfactants onto polyvinyl alcohol as hydrophobic polymer surface. J Dispersion Sci Technol 31(8):1091–1099. https://doi.org/10.1080/01932690903224581
Zargar V, Asghari M et al (2015) A review on chitin and chitosan polymers: Structure, chemistry, solubility, derivatives, and applications. ChemBioEng Reviews 2(3):204–226. https://doi.org/10.1002/cben.201400025
Pirbazari A, Pargami N et al (2015) Surfactant-coated tea waste: Preparation, characterization and its application for methylene blue adsorption from aqueous solution. J Environ Anal Toxicol 5(5):1. https://doi.org/10.4172/2161-0525.1000310
Kushwaha AK, Gupta N et al (2014) Removal of cationic methylene blue and malachite green dyes from aqueous solution by waste materials of daucus carota. J Saudi Chem Soc 18(3):200–207. https://doi.org/10.1016/j.jscs.2011.06.011
Choo KW, Ching YC et al (2016) Preparation and characterization of polyvinyl alcohol-chitosan composite films reinforced with cellulose nanofiber. Materials 9:644–652
Kenawy E-R, Kamoun EA et al (2014) Physically crosslinked poly (vinyl alcohol)-hydroxyethyl starch blend hydrogel membranes: Synthesis and characterization for biomedical applications. Arab J Chem 7(3):372–380. https://doi.org/10.1016/j.arabjc.2013.05.026
Wan Ngah WS, Teong LC et al (2011) Adsorption of dyes and heavy metal ions by chitosan composites: A review. Carbohydr Polym 83(4):1446–1456. https://doi.org/10.1016/j.carbpol.2010.11.004
Zendehdel M, Barati A et al (2010) Removal of methylene blue dye from wastewater by adsorption onto semi-inpenetrating polymer network hydrogels composed of acrylamide and acrylic acid copolymer and polyvinyl alcohol. J Environ Health Sci Eng 7(5):431–436. https://doi.org/10.1016/j.biortech.2007.05.028
Osasona I, Faboya OL et al (2013) Kinetic, equilibrium and thermodynamic studies of the adsorption of methylene blue from synthetic wastewater using cow hooves. Br J Appl Sci Technol 3(4):1006–1021. https://doi.org/10.9734/BJAST/2014/3695
Luo JY, Lin YR et al (2015) Controllable dye adsorption behavior on amorphous tungsten oxide nanosheet surfaces. RSC Adv 5(122):100898–100904. https://doi.org/10.1039/C5RA18601C
Banerjee S, Gautam RK et al (2015) Rapid scavenging of methylene blue dye from a liquid phase by adsorption on alumina nanoparticles. RSC Adv 5(19):14425–14440
Kumar M, Tripathi BP et al (2009) Crosslinked chitosan/polyvinyl alcohol blend beads for removal and recovery of cd (ii) from wastewater. J Hazard Mater 172(2):1041–1048. https://doi.org/10.1016/j.jhazmat.2009.07.108
Sanchez LM, Ollier RP et al (2019) Sorption behavior of polyvinyl alcohol/bentonite hydrogels for dyes removal. J Polym Res 26(6):142
Halsey GD (1952) The role of surface heterogeneity in adsorption. Adv Catal 4:259–269. https://doi.org/10.1016/S0360-0564(08)60616-1
Nassar MY, Abdallah S (2016) Facile controllable hydrothermal route for a porous comn2o4 nanostructure: Synthesis, characterization, and textile dye removal from aqueous media. RSC Adv 6(87):84050–84067. https://doi.org/10.1039/C6RA12424K
Hamdaoui O, Naffrechoux E (2007) Modeling of adsorption isotherms of phenol and chlorophenols onto granular activated carbon: Part i. Two-parameter models and equations allowing determination of thermodynamic parameters. J Hazard Mater 147(1):381–394. https://doi.org/10.1016/j.jhazmat.2007.01.021
Wang N, Chang PR et al (2014) Graphene–poly (vinyl alcohol) composites: Fabrication, adsorption and electrochemical properties. Appl Surf Sci 314:815–821. https://doi.org/10.1016/j.apsusc.2014.07.075
Saad N, Al-Mawla M et al (2015) Surface-functionalized silica aerogels and alcogels for methylene blue adsorption. RSC Adv 5(8):6111–6122. https://doi.org/10.1039/C4RA15504A
Baraka A (2012) Adsorptive removal of tartrazine and methylene blue from wastewater using melamine-formaldehyde-tartaric acid resin (and a discussion about pseudo second order model). Desalin Water Treat 44(1–3):128–141. https://doi.org/10.1080/19443994.2012.691778
Paşka OM, Păcurariu C et al (2014) Kinetic and thermodynamic studies on methylene blue biosorption using corn-husk. RSC Adv 4(107):62621–62630. https://doi.org/10.1039/C4RA10504D
Ma J, Jia Y et al (2012) Kinetics and thermodynamics of methylene blue adsorption by cobalt-hectorite composite. Dyes Pigments 93(1):1441–1446. https://doi.org/10.1016/j.dyepig.2011.08.010
Ling YW, Ng CA et al (2015) Membrane bioreactor performance improvement by adding adsorbent and coagulant: A comparative study. Desalination and Water Treatment. https://doi.org/10.1080/19443994.2015.1060900,1-7
Öztürk A, Malkoc E (2014) Adsorptive potential of cationic basic yellow 2 (by2) dye onto natural untreated clay (nuc) from aqueous phase: Mass transfer analysis, kinetic and equilibrium profile. Appl Surf Sci 299:105–115. https://doi.org/10.1016/j.dyepig.2011.08.010
Tan BK, Ching YC et al (2015) A Review of Natural Fiber Reinforced Poly (Vinyl Alcohol) Based Composites: Application and Opportunity. Polymers 7(11):2205–2222
El-Araby HA, Ibrahim AMMA et al (2017) Sesame husk as adsorbent for copper (ii) ions removal from aqueous solution. GEP 5(7):109. https://doi.org/10.4236/gep.2017.57011
Hema M, Arivoli S (2008) Adsorption kinetics and thermodynamics of malachite green dye unto acid activated low cost carbon. J Appl Sci Environ Manage 12 (1). doi:DOI:https://doi.org/10.4314/jasem.v12i1.55568
Zhao R, Wang Y et al (2015) Water-insoluble sericin/β-cyclodextrin/pva composite electrospun nanofibers as effective adsorbents towards methylene blue. Colloids Surf B Biointerfaces 136:375–382. https://doi.org/10.1016/j.colsurfb.2015.09.038
Ghahremani R, Baheri B et al (2015) Novel crosslinked and zeolite-filled polyvinyl alcohol membrane adsorbents for dye removal. Res Chem Intermed 41(12):9845–9862
Nesic AR, Velickovic SJ et al (2014) Novel composite films based on amidated pectin for cationic dye adsorption. Colloids Surf B Biointerfaces 116:620–626. https://doi.org/10.1016/j.colsurfb.2013.10.031
Mok CF, Ching YC et al (2020) Adsorption of dyes using poly (vinyl alcohol)(PVA) and pva-based polymer composite adsorbents: A review. J Polym Environ:1–19. https://doi.org/https://doi.org/10.1007/s10924-020-01656-4
Sabarish R, Unnikrishnan G (2018) Polyvinyl alcohol/carboxymethyl cellulose/zsm-5 zeolite biocomposite membranes for dye adsorption applications. Carbohydr Polym 199:129–140
Dai H, Huang Y et al (2018) Eco-friendly polyvinyl alcohol/carboxymethyl cellulose hydrogels reinforced with graphene oxide and bentonite for enhanced adsorption of methylene blue. Carbohydr Polym 185:1–11
Kong Y, Zhuang Y et al (2019) Dye removal by eco-friendly physically cross-linked double network polymer hydrogel beads and their functionalized composites. J Environ Sci 78:81–91
Ma Y-X, Li X et al (2020) Fabrication of 3d porous polyvinyl alcohol/sodium alginate/graphene oxide spherical composites for the adsorption of methylene blue. J Nanosci Nanotechnol 20(4):2205–2213
Acknowledgements
The authors would like to acknowledge the financial support from the University Malaya research grant: ST017-2018, RU019O-2017 and GPF 002A-2019 for the success of this project.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mok, C.F., Ching, Y.C., Osman, N.A.A. et al. Adsorbents for removal of cationic dye: nanocellulose reinforced biopolymer composites. J Polym Res 27, 373 (2020). https://doi.org/10.1007/s10965-020-02347-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-020-02347-3