Abstract
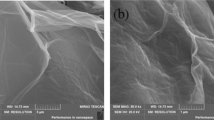
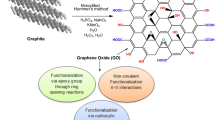
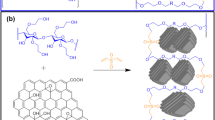
Starch-g-polyacrylamide copolymer and its nanocomposite with Fe3O4 and graphene oxide have been successfully synthesized. The synthesized samples were characterized using FTIR, XRD, SEM, HRTEM, and VSM. The factors that affect the adsorption efficiency of samples for Ni(II) ions from aqueous solutions as pH, initial Ni(II) ions concentration, contact time and temperature have been examined. The maximum adsorption capacities achieved by the synthesized samples were 195 mg g−1 and 290 mg g−1 for starch-g-polyacrylamide copolymer and its starch-g-polyacrylamide/ Fe3O4/ graphene oxide nanocomposite respectively. Kinetic studies showed that the adsorption was well described by the pseudo–second-order model and the equilibrium adsorption data fitted Freundlich model. Thermodynamic studies showed that the adsorption capacity increases as temperature increase up to 313 K but higher temperatures result in dissolution of starch. Results showed that the adsorption process is spontaneous, endothermic in nature and leads to a greater entropy.










Similar content being viewed by others
References
Jain M, Kumar Garg V, Kadirvelu K (2013) Removal of Ni(II) from aqueous system by chemically modified sunflower biomass. Desalin Water Treat 52:5681–5695
Nirav PR, Prapti US, Nisha KS (2016) Adsorptive removal of nickel (II) ions from aqueous environment: A review. J Environ Manag 179:1–20
Jong-Hwan P, Yong SO, Seong-Heon K, Ju-Sik Ch, Jong-Soo H, Ronald DD, Dong-Cheol S (2016) Competitive adsorption of heavy metals onto sesame straw biochar in aqueous solutions. Chemosphere 142:77–83
Zafar MN, Aslam I, Nadeem R, Munir S, Rana UA, Khan SU-D (2015) Characterization of chemically modified biosorbents from rice bran for biosorption of Ni(II). J Taiwan Inst Chem Eng 46:82–88
Keranen A, Leivisk T, Salakka A, Tanskanen J (2015) Removal of nickel and vanadium from ammoniacal industrial wastewater by ion exchange and adsorption on activated carbon. Desalin Water Treat 53:2645–2654
Lalsing PK (2019) Rapid removal of nickel (II) by coconut leaves powder as bioadsorbent. J Adv Chem Sci 5:632–636
Dhanashree C, Vikesh G, Virendra K (2014) Adsorptive removal of copper(II) from aqueous solution onto the waste sweet lime peels (SLP): equilibrium, kinetics and thermodynamics studies. J Desalin Water Treat 52:7822–7837
Wu F-C, Tseng R-L, Juang R-S (2010) A review and experimental verification of using chitosan and its derivatives as adsorbents for selected heavy metals. J Environ Manage 91:798–806
Ali O, Mohamed SK (2017) Adsorption of copper ions and alizarin red S from aqueous solutions onto a polymeric nanocomposite in single and binary systems. Turkish J Chem 41:967–986
Abou El-Reash YG, Abdelghany AM, Lepold K (2017) Solid phase extraction of Cu2+ and Pb2+ from water using new thermally treated Chitosan/ Polyacrylamide thin films; Adsorption kinetics and thermodynamics. Int J Environ Ana Chem 97:965–982
Esmat M, Farghali AA, Khedr MH, El-Sherbiny IM (2017) Alginate-Based Nanocomposites for Efficient Removal of Heavy Metal Ions. Int J Bio Macromol 102:272–283
Niu HY, Pu LM, Li J (2015) Removal of Cu (II) Ions by a novel starch-based adsorbent from aqueous solution. App Mechanics Mat 713:2815–2818
Ibrahim BM, Fakhre NA (2019) Crown ether modification of starch for adsorption of heavy metals from synthetic wastewater. Int J Biolog Macromolecules 123:70–80
Naushad M, Ahamad T, Sharma G, Ala’a H, Albadarin AB, Alam MM, Alothman ZA, Alshehri SM, Ghfar AA (2016) Synthesis and characterization of a new starch/SnO2 nanocomposite for efficient adsorption of toxic Hg2+ metal ion. Chem Eng J 300:306–316
Hayeeye F, Yu QJ, Sattar M, Chinpa W, Sirichote O (2018) Adsorption of Pb2+ ions from aqueous solutions by gelatin/activated carbon composite bead form. Adsorp Sci Techn 36:355–371
Baseri H, Tizro S (2017) Treatment of nickel ions from contaminated water by magnetite based nanocomposite adsorbents: Effects of thermodynamic and kinetic parameters and modeling with Langmuir and Freundlich isotherms. Pro Safety Env Pro 109:465–477
Ekebafe LO, Ogbeifun DE, Okieimen FE (2012) Removal of heavy metals from aqueous media using native cassava starch hydrogel. African J Env Sci Techn 6:275–282
Feng K, Wen G (2017) Absorbed Pb2+ and Cd2+ ions in water by cross-linked starch xanthate. Int J Polym Sci. https://doi.org/10.1155/2017/6470306
Sancey B, Trunfio GA, Crini G (2011) Heavy metal removal from industrial effluents by sorption on cross-linked starch: chemical study and impact on water toxicity. J Env manag 92:765–772
Abdul-Raheim ARM, El-Saeed SM, Farag RK, Abdel-Raouf ME (2016) Low cost biosorbents based on modified starch iron oxide nanocomposites for selective removal of some heavy metals from aqueous solutions. Adv Mater Lett 7(5):402–409
Massart R (1981) Preparation of aqueous magnetic liquids in alkaline and acidic media. IEEE Trans Magn 17:1247–1248
Mohamed SK, Hegazy SH, Abdelwahab NA, Ramadan AM (2018) Coupled adsorption-photocatalytic degradation of crystal violet under sunlight using chemically synthesized grafted sodium alginate/ZnO/graphene oxide composite. Int J Bio Macrom 108:1185–1198
Hariani PL, Faizal M, Marsi R, Setiabudidaya D (2013) Synthesis and properties of Fe3O4 nanoparticles by co-precipitation method to removal procion dye. Int J Env Sci Develop 4:336–340
Kassaee MZ, Motamedi E, Majdi M (2011) Magnetic Fe3O4- graphene oxide/polystyrene: Fabrication and characterization of a promising nanocomposite. Chem Eng J 172:540–549
Cheng X, Kumar V, Yokozeki T, Goto T, Takahashi T, Koyanagi J, Wu L, Wang R (2016) Highly conductive graphene oxide/polyaniline hybrid polymer nanocomposites with simultaneously improved mechanical properties. Composites A 82:100–107
Wang S, Zhang C, Chang Q (2017) Synthesis of magnetic crosslinked starch-graft-poly(acrylamide)-co-sodium xanthate and its application in removing heavy metal ions. J Exp Nanosci 12:270–284
Hosseinzadeh H, Ramin S (2015) Magnetic and pH-responsive starch-g-poly(acrylic acid-coacrylamide)/ graphene oxide superabsorbent nanocomposites: one-pot synthesis, characterization and swelling behavior. Starch 68:200–212
Rattana T, Chaiyakun S, Witit-anun N, Nuntawong N, Chindaudom P, Oaew S, Kedkeaw C, Limsuwan P (2012) Preparation and characterization of graphene oxide Nanosheets. Procedia Eng 32:759–764
Hoogmoed CG, Busscher HJ, Vos P (2003) Fourier transform infrared spectroscopy studies of alginate–PLL capsules with varying compositions. J Biomed mat res 67A:172–178
Zubir NA, Yacou C, Motuzas J, Zhang X, da Costa JCD (2014) Structural and Functional Investigation of Graphene Oxide-Fe3O4 nanocomposites for the Heterogeneous Fenton-Like Reaction. Sci Rep 4:4594–4601
Soleymani M, Akbari A, Mahdavinia GR (2018) Magnetic PVA/laponite RD hydrogel nanocomposites for adsorption of model protein BSA. Polym Bull 76:2321–2340
Wu L, Ye Y, Liu F, Tan C, Liu H, Wang S, Wang J, Yi W, Wu W (2013) Organo-bentonite-Fe3O4 poly(sodium acrylate) magnetic superabsorbent nanocomposite: Synthesis, characterization, and Thorium(IV) adsorption. Appl Clay Sci 83:405–414
Mohamed SK, Alazhary AM, Al-Zaqri N, Alsalme A, Alharthi FA, Hamdy MS (2020) Cost-effective adsorbent from arabinogalactan and pectin of cactus pear peels: Kinetics and thermodynamics studies. Int J Biolog Macromol 150:941–947
Dabrowski A (2001) Adsorption—From Theory to Practice. Adv Colloid Interface Sci 93:135–224
Adamson AW, Gast AP (1997) Physical Chemistry of Surfaces, sixth ed., Wiley– Interscience, New York
Freundlich H (1906) Ueber die adsorption in loesungen. Zeitschrift für Physikalische Chemie 57:385–470
Ayawei N, Ebelegi AN, Wankasi D (2017) Modelling and Interpretation of Adsorption Isotherms. J Chem. https://doi.org/10.1155/2017/3039817
Simi CK, Abraham TE (2007) Hydrophobic grafted and crosslinked starch nanoparticles for drug delivery. Bioprocess Biosyst Eng 30:173–180
Dawodu F, Akpomie K (2014) Simultaneous adsorption of Ni(II) and Mn(II) ions from aqueous solution unto a Nigerian kaolinite clay. J Mater Res Tech 3:129–141
Abdelrahman EA, Abdel-Salam ET, El Rayes SM, Mohamed NS (2019) Facile synthesis of graft copolymers of maltodextrin and chitosan with 2-acrylamido-2-methyl-1-propanesulfonic acid for efficient removal of Ni(II), Fe(III), and Cd(II) ions from aqueous media. J Polym Res. https://doi.org/10.1007/s10965-019-1920-4
Bartczak P, Norman M, Klapiszewski L, Karwanska N, Kawalec M, Baczynska M, Wysokowski M, Zdarta J, Ciesielczyk F, Jesionowski T (2018) Removal of nickel(II) and lead(II) ions from aqueous solution using peat as a low-cost adsorbent: A kinetic and equilibrium study. Arabian J Chem 11:1209–1222
Singh H, Rattan V (2011) Adsorption of nickel from aqueous solutions using low cost biowaste adsorbents. Water Quality Res J Canada 46:239–249
Luyen TT, Hoang VT, Thu DL, Giang LB, Lam DT (2019) Studying Ni(II) adsorption of magnetite/graphene oxide/chitosan nanocomposite. Adv polym tech. https://doi.org/10.1155/2019/8124351
Samadi N, Hasanzadeh R, Rasad M (2015) Adsorption isotherms, kinetic, and desorption studies on removal of toxic metal ions from aqueous solutions by polymeric adsorbent. J Appl Polym Sci 132:41642
Ji F, Li C, Tang B, Xu J, Lu G, Liu P (2012) Preparation of cellulose acetate/zeolite composite fiber and its adsorption behavior for heavy metal ions in aqueous solution. Chem Eng J 209:325–333
Cegłowskia M, Gierczyka B, Frankowskia M, Popenda L (2018) A new low-cost polymeric adsorbents with polyamine chelating groups for efficient removal of heavy metal ions from water solutions. Reac Func Polym 131:64–74
Kushwaha AK, Gupta N, Chattopadhyaya MC (2017) Dynamics of adsorption of Ni(II) Co(II) and Cu(II) from aqueous solution onto newly synthesized poly[N-(4-[4-(aminophenyl)methylphenylmethacrylamide])]. Arabian J Chem 10:S1645–S1653
He H, Feng Q (2017) Preparation and application of Ni(II) ion-imprinted silica gel polymer for selective separation of Ni(II) from aqueous solution. Roy soc chem 7:15102–15111
Zhang X, Wang X (2015) Adsorption and Desorption of Nickel(II) Ions from Aqueous Solution by a Lignocellulose/Montmorillonite Nanocomposite. PLoS ONE 10:e0117077
Acknowledgements
The authors are grateful to Prof. Soad Ashry, the professor at the national research center for the Atomic Absorption facilities. The authors also thank, Professor Mohamed Rashad, the Central Metallurgical Research and Development Institute for the VSM measurements.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hegazy, S.H., Mohamed, S.K. Starch-graft-polyacrylamide copolymer /Fe3O4 /graphene oxide nanocomposite: synthesis, characterization, and application as a low-cost adsorbent for Ni (II) from aqueous solutions. J Polym Res 28, 49 (2021). https://doi.org/10.1007/s10965-020-02275-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-020-02275-2