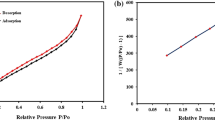
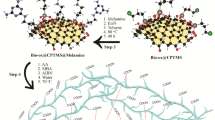
Abstract
Here, ethylenediamine-epichlorohydrin-trichlorophenol (EET) cross-linked polymer was synthesized and characterized by Fourier Transforms Infrared spectroscopy (FTIR), thermogravimetric analysis (TGA–DSC) and scanning electron microscopy (SEM). EET exhibited substantial antibacterial activity with inhibition zones of 38 and 64 mm against E. coli and S. aureus bacteria. Therefore, it was applied to treat methyl orange (MO) and rhodamine B (RB) dyes containing synthetic aqueous solutions under varying operation parameters. Notably, 10 and 15 mg of EET removed 98.72% of MO at pH 8 and 92.45% of RB at pH 3. Moreover, EET cross-linked polymer retained stable activities of about 98.6% over five consecutive recycling runs for MO dye. The EET demonstrated a fast adsorption rate and the adsorption data fits well with the pseudo-second-order for both dyes, suggesting chemisorption. Also, considering the correlation coefficient values, the experimental dataset fits suitably with Temkin equation for RB and Langmuir equation for MO. Thermodynamic evaluations for both dyes show spontaneity onto the cross-linked polymer.

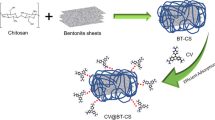
Graphical abstract












Similar content being viewed by others
References
Ratnamala GM, Shetty KV, Srinikethan G (2012) Removal of remazol brilliant blue dye from dye-contaminated water by adsorption using red mud: equilibrium, kinetic, and thermodynamic studies. Water Air Soil Pollut 223(9):6187–6199
Gong R, Li M, Yang C, Sun Y, Chen J (2005) Removal of cationic dyes from aqueous solution by adsorption on peanut hull. J Hazard Mater 121(1–3):247–250
Chequer FMD, de Oliveira GAR, Ferraz ERA, Cardoso JC, Zanoni MVB, de Oliveira DP (2013) Textile dyes: dyeing process and environmental impact. Eco-friendly Text Dye Finish 6:151–176
Jain R, Sikarwar S (2006) Photocatalytic and adsorption studies on the removal of dye Congo red from wastewater. Int J Environ Pollut 27(1–3):158–178
Koprivanac N, Kušić H (2009) Hazardous organic pollutants in colored wastewaters. Nova Science Publishers
Mishra G, Tripathy M (1993) A critical review of the treatment for decolorization of dye wastewater. Colourage 40:35–38
Tahir H, Sultan M, Akhtar N, Hameed U, Abid T (2016) Application of natural and modified sugar cane bagasse for the removal of dye from aqueous solution. J Saudi Chem Soc 20:S115–S121
Hassanzadeh-Tabrizi SA, Motlagh MM, Salahshour S (2016) Synthesis of ZnO/CuO nanocomposite immobilized on γ-Al2O3 and application for removal of methyl orange. Appl Surf Sci 384:237–243
Moazami A, Montazer M, Dolatabadi MK (2018) Rapid discoloration of methyl orange in water by conductive cu/Cu2O/rGO modified polyester fabric. J Polym Environ 26(6):2502–2513
Xin Q, Fu J, Chen Z, Liu S, Yan Y, Zhang J, Xu Q (2015) Polypyrrole nanofibers as a high-efficient adsorbent for the removal of methyl orange from aqueous solution. J Environ Chem Eng 3(3):1637–1647
Oladipo AA, Ifebajo AO, Nisar N, Ajayi OA (2017) High-performance magnetic chicken bone-based biochar for efficient removal of rhodamine-B dye and tetracycline: competitive sorption analysis. Water Sci Technol 76(2):373–385
Geng T-M, Wu D-Y, Huang W, Huang R-Y, Wu G-H (2014) Fluorogenic detection of Hg2+, Cd2+, Fe2+, Pb2+ cations in aqueous media by means of an acrylamide-acrylic acid copolymer chemosensor with pendant rhodamine-based dyes. J Polym Res 21(3):354–362
Imam SS, Babamale HF (2020) A short review on the removal of rhodamine b dye using agricultural waste-based adsorbents. AJOCS 7(1):25–37
Kadirvelu K, Karthika C, Vennilamani N, Pattabhi S (2005) Activated carbon from industrial solid waste as an adsorbent for the removal of rhodamine-B from aqueous solution: kinetic and equilibrium studies. Chemosphere 60(8):1009–1017
Crini G, Lichtfouse E, Wilson LD, Morin-Crini N (2018) Adsorption-oriented processes using conventional and non-conventional adsorbents for wastewater treatment. In: Green Adsorbents for Pollutant Removal. Springer 23–71
Saberi A, Alipour E, Sadeghi M (2019) Superabsorbent magnetic Fe3O4-based starch-poly (acrylic acid) nanocomposite hydrogel for efficient removal of dyes and heavy metal ions from water. J Polym Res 26(12):271–285
Munagapati VS, Yarramuthi V, Kim DS (2017) Methyl orange removal from aqueous solution using goethite, chitosan beads and goethite impregnated with chitosan beads. J Mol Liq 240:329–339
Kaushal M, Tiwari A (2010) Removal of rhodamine-B from aqueous solution by adsorption onto crosslinked alginate beads. J Dispers Sci Technol 31(4):438–441
Biçaak N, Şenkal BF (1998) Removal of nitrite ions from aqueous solutions by cross-linked polymer of ethylenediamine with epichlorohydrin. React Funct Polym 36(1):71–77
Sun C, Zhang X, Zhang Z, Zhang Y (2013) Color removal from dyeing wastewater by the polymer of epichlorohydrin- ethylenediamine. Adv Mater Res 752:1448–1451
Molinari R, Argurio P, Poerio T (2004) Comparison of polyethylenimine, polyacrylic acid and poly(dimethylamine-co-epichlorohydrin-co-ethylenediamine) in Cu2+ removal from wastewaters by polymer-assisted ultrafiltration. Desalination 162(1–3):217–228
Chunhui D, Xinyi Z, Xumin M (2020) The surface tunability and dye separation property of PVDF porous membranes modified by P(MMA-b-MEBIm-Br): effect of poly (ionic liquid) brush lengths. J Polym Res 29(79):1–11
Nendza M, Seydel JK (1990) Application of bacterial growth kinetics to in vitro toxicity assessment of substituted phenols and anilines. Ecotoxicol Environ Saf 19(2):228–241
Michałowicz J, Duda W (2007) Phenols - sources and toxicity. Pol J Environ Stud 16(3):347–362
Oladipo AA, Gazi M (2015) Two-stage batch sorber design and optimization of biosorption conditions by Taguchi methodology for the removal of acid red 25 onto magnetic biomass. Korean J Chem Eng 32(9):1864–1878
Alkan M, Doğan M (2001) Adsorption of copper (II) onto perlite. J Colloid Interface Sci 243(2):280–291
Nakhjiri MT, Marandi GB, Kurdtabar M (2018) Effect of bis [2-(methacryloyloxy) ethyl] phosphate as a crosslinker on poly (AAm-co-AMPS)/Na-MMT hydrogel nanocomposite as potential adsorbent for dyes: kinetic, isotherm and thermodynamic study. J Polym Res 25:244–263
Alokour M, Yilmaz E (2019) Photoinitiated synthesis of poly (poly (ethylene glycol) methacrylate-co-diethyl amino ethyl methacrylate) superabsorbent hydrogels for dye adsorption. J Appl Polym Sci 136(26):47707–47724
Kennedy MV, Stojanovic BJ, Shuman JFL (1972) Chemical and thermal aspects of pesticide disposal. J Environ Qual 1:63–65
Segal L, Eggerton FV (1961) Infrared spectra of ethylenediamine and the dimethylethylenediamines. Appl Spectrosc 15(4):116–117
Jiao M, Yang K, Cao J, Diao Q, Zhang W, Yu M (2016) Influence of epichlorohydrin content on structure and properties of high-ortho phenolic epoxy fibers. J Appl Polym Sci 133(18):43375–43381
González MG, Cabanelas JC, Baselga J (2012) Applications of FTIR on epoxy resins-identification, monitoring the curing process, phase separation and water uptake. Infrared Spectrosc Sci Eng Technol 2:261–284
Zhan C, Chen F, Dai H, Yang J, Zhong M (2013) Photocatalytic activity of sulfated Mo-doped TiO2@fumed SiO2 composite: a mesoporous structure for methyl orange degradation. Chem Eng J 225:695–703
Oladipo AA, Ifebajo AO (2018) Highly efficient magnetic chicken bone biochar for removal of tetracycline and fluorescent dye from wastewater: two-stage adsorber analysis. J Environ Manag 209:9–16
Umpuch C, Sakaew S (2013) Removal of methyl orange from aqueous solutions by adsorption using chitosan intercalated montmorillonite. J Sci Technol 35(4):451–459
Li Q, Tang X, Sun Y, Wang Y, Long Y, Jiang J, Xu H (2015) Removal of rhodamine B from wastewater by modified volvariella volvacea: batch and column study. RSC Adv 5(32):25337–25347
Venkatraman BR, Gayathri U, Elavarasi S, Arivoli S (2012) Removal of rhodamine B dye from aqueous solution using the acid activated cynodon dactylon carbon. Der Chem Sin 3(1):99–113
Bazrafshan E, Zarei AA, Nadi H, Zazouli MA (2014) Adsorptive removal of methyl orange and reactive red 198 dyes by moringa peregrina ash. Indian J Chem Technol 21(2):105–113
Türkcan C, Uygun DA, Akgöl S, Denizli A (2014) Reactive red 120 and NI (II) derived poly (2-hydroxyethyl methacrylate) nanoparticles for urease adsorption. J Appl Polym Sci 131(2):39757–39764
Al-Ghouti MA, Da'ana DA (2020) Guidelines for the use and interpretation of adsorption isotherm models: a review. J Hazard Mater 393:1223834–1223856
Paiwei L, Xin D, Qilin Y, Zhenxi W, Shangxi Z, Meng C (2020) A novel modification method for polystyrene microspheres with dithizone and the adsorption properties for Pb2+. J Polym Res 27(195):1–10
Bahrudin NN, Nawi MA, Ismail WINW (2018) Physical and adsorptive characterizations of immobilized polyaniline for the removal of methyl orange dye. Korean J Chem Eng 35(7):1450–1461
Allouche F-N, Yassaa N, Lounici H (2015) Sorption of methyl orange from aqueous solution on chitosan biomass. Procedia Earth Planet Sci 15:596–601
Hou X-X, Deng Q-F, Ren T-Z, Yuan Z-Y (2013) Adsorption of Cu2+ and methyl orange from aqueous solutions by activated carbons of corncob-derived char wastes. Environ Sci Pollut Res 20(12):8521–8534
Robati D, Mirza B, Rajabi M et al (2016) Removal of hazardous dyes-BR 12 and methyl orange using graphene oxide as an adsorbent from aqueous phase. Chem Eng J 284:687–697
Allouche F-N, Yassaa N, Lounici H (2015) Sorption of methyl orange from aqueous solution on chitosan biomass. Procedia Earth Planet Sci 15:596–601
Umpuch C, Sakaew S (2013) Removal of methyl orange from aqueous solutions by adsorption using chitosan intercalated montmorillonite. Songklanakarin J Sci Technol 35(4):451–459
Bahrudin NN, Nawi MA, Ismail WINW (2018) Physical and adsorptive characterizations of immobilized polyaniline for the removal of methyl orange dye. Korean J Chem Eng 35(7):1450–1461
Thakur A, Kaur H (2017) Response surface optimization of Rhodamine B dye removal using paper industry waste as adsorbent. Int J Ind Chem 8(2):175–186
Arslan M, Günay K (2018) Synthesis and use of PET fibers grafted with 4-vinyl pyridine and 2-methylpropenoic acid for removal of rhodamine B and methylene blue from aqueous solutions. J Polym Sci Appl 2(1):2
Wang M, Fu J, Zhang Y et al (2015) Removal of rhodamine B, a cationic dye from aqueous solution using poly (cyclotriphosphazene-co-4, 4′-sulfonyldiphenol) nanotubes. J Macromol Sci Part A 52(2):105–113
Damiyine B, Guenbour A, Boussen R (2017) Rhodamine B adsorption on natural and modified moroccan clay with cetyltrimethylammonium bromide: Kinetics, equilibrium and thermodynamics. J Mater Environ Sci 8(3):860–871
Saini J, Garg VK, Gupta RK, Kataria N (2017) Removal of Orange G and Rhodamine B dyes from aqueous system using hydrothermally synthesized zinc oxide loaded activated carbon (ZnO-AC). J Environ Chem Eng 5(1):884–892
Acknowledgements
We thank Dr. Fadwa Odeh (The University of Jordan, Chemistry Department) and Dr. Walhan Alshaer (The University of Jordan, Cell Therapy Center) for helping us to characterize our polymer. The authors acknowledge the suggestions, editing and professional support received during manuscript curation and revision from Associate Professor Akeem Oladipo (Chemistry Department of Eastern Mediterranean University).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mustafa, F.S., Güran, M. & Gazi, M. Effective removal of dyes from aqueous solutions using a novel antibacterial polymeric adsorbent. J Polym Res 27, 247 (2020). https://doi.org/10.1007/s10965-020-02227-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-020-02227-w