Abstract
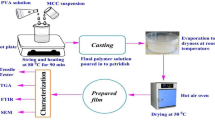
Cellulose was isolated from waste coconut husk (Cocos nucifera) via alkali and single bleaching treatment. The isolated cellulose (CH-A-1B) was found to have residual lignin which imparts amorphous character to the polymer matrix as confirmed by X-ray diffraction analysis (XRD). Fourier transform infrared (FTIR) peak at 1507 cm−1 confirmed the presence of lignin and the appearance of three new peaks at 1320, 1416, and 1595 cm−1 showed successful attachment of carboxymethyl group onto the cellulose backbone. Carboxymethylation of cellulose from CH-A-1B produced carboxymethyl cellulose (CMC) with lower crystallinity compared to that of the double bleaching treatment cellulose (CH-A-2B). The CMC film prepared by solvent-casting method from CH-A-1B using 38 wt% NaOH possessed the highest conductivity of σ = 4.82 × 10−4 mS cm−1. The electrochemical properties of the CMC polymer electrolyte and the degree of crystallinity of the polymer matrix were found to be influenced by the amount of lignin content which acts as a natural plasticizer.










Similar content being viewed by others
Change history
02 December 2020
The original version of this article unfortunately contained many mistakes with regard to some mathematical expressions and to Tables 3 & 5.
Abbreviations
- ATR:
-
attenuated total reflectance
- CC:
-
commercial cellulose
- CH:
-
coconut husk
- CI:
-
crystallinity index
- CMC:
-
carboxymethyl cellulose
- CML:
-
carboxymethyl lignin
- CPE:
-
constant phase element
- EIS:
-
electrochemical impedance
- FTIR:
-
Fourier-transform infrared spectroscopy
- LE:
-
liquid electrolyte
- MCA:
-
monochloroacetic acid
- SPE:
-
solid polymer electrolyte
- XRD:
-
X-ray diffractometry
References
Deshpande R, Pawar O, Kute A (2017) Advancement in the technology of organic light emitting diodes. In: 2017 international conference on innovations in information, embedded and communication systems (ICIIECS), 2017. IEEE, pp 1-5. https://doi.org/10.1109/ICIIECS.2017.8276054
Chakraborty S, Sadhu P, Goswami U (2016) Barriers in the advancement of solar energy in developing countries like India. Problemy Ekorozwoju – Prob Sustain Dev 11(2):75–80
Zhang Z, Zhang X, Chen W, Rasim Y, Salman W, Pan H, Yuan Y, Wang C (2016) A high-efficiency energy regenerative shock absorber using supercapacitors for renewable energy applications in range extended electric vehicle. Appl Energy 178:177–188. https://doi.org/10.1016/j.apenergy.2016.06.054
Rajarathnam G, Easton M, Schneider M, Masters A, Maschmeyer T, Vassallo A (2016) The influence of ionic liquid additives on zinc half-cell electrochemical performance in zinc/bromine flow batteries. RSC Adv 6(33):27788–27797. https://doi.org/10.1039/C6RA03566C
Yang Z, Ren J, Zhang Z, Chen X, Guan G, Qiu L, Zhang Y, Peng H (2015) Recent advancement of nanostructured carbon for energy applications. Chem Rev 115(11):5159–5223. https://doi.org/10.1021/cr5006217
Dudney N (2000) Addition of a thin-film inorganic solid electrolyte (Lipon) as a protective film in lithium batteries with a liquid electrolyte. J Power Sources 89(2):176–179. https://doi.org/10.1016/S0378-7753(00)00427-4
Abe T, Sagane F, Ohtsuka M, Iriyama Y, Ogumi Z (2005) Lithium-ion transfer at the interface between lithium-ion conductive ceramic electrolyte and liquid electrolyte-a key to enhancing the rate capability of lithium-ion batteries. J Electrochem Soc 152(11):A2151–A2154. https://doi.org/10.1149/1.2042907
Zhang S (2007) A review on the separators of liquid electrolyte Li-ion batteries. J Power Sources 164(1):351–364. https://doi.org/10.1016/j.jpowsour.2006.10.065
Aurbach D, Zinigrad E, Cohen Y, Teller H (2002) A short review of failure mechanisms of lithium metal and lithiated graphite anodes in liquid electrolyte solutions. Solid State Ionics 148(3–4):405–416. https://doi.org/10.1016/S0167-2738(02)00080-2
Smitha B, Sridhar S, Khan A (2005) Solid polymer electrolyte membranes for fuel cell applications—a review. J Membr Sci 259(1–2):10–26. https://doi.org/10.1016/j.memsci.2005.01.035
Savadogo O (1998) Emerging membrane for electrochemical systems: (I) solid polymer electrolyte membranes for fuel cell systems. J New Mater Electrochem Syst 1(1):47–66
Abraham K, Alamgir M (1990) Li+-conductive solid polymer electrolytes with liquid-like conductivity. J Electrochem Soc 137(5):1657–1658
Murata K, Izuchi S, Yoshihisa Y (2000) An overview of the research and development of solid polymer electrolyte batteries. Electrochim Acta 45(8–9):1501–1508. https://doi.org/10.1016/S0013-4686(99)00365-5
Nguyen C, Argun A, Hammond P, Lu X, Lee P (2011) Layer-by-layer assembled solid polymer electrolyte for electrochromic devices. Chem Mater 23(8):2142–2149. https://doi.org/10.1021/cm103572q
Li M, Wang X, Yang Y, Chang Z, Wu Y, Holze R (2015) A dense cellulose-based membrane as a renewable host for gel polymer electrolyte of lithium ion batteries. J Membr Sci 476:112–118. https://doi.org/10.1016/j.memsci.2014.10.056
Singh P, Nagarale R, Pandey S, Rhee H, Bhattacharya B (2011) Present status of solid state photoelectrochemical solar cells and dye sensitized solar cells using PEO-based polymer electrolytes. Adv Nat Sci-Nanosci 2(2):023002. https://doi.org/10.1088/2043-6262/2/2/023002
Rani M, Rudhziah S, Ahmad A, Mohamed N (2014) Biopolymer electrolyte based on derivatives of cellulose from kenaf bast fiber. Polymers 6(9):2371–2385. https://doi.org/10.3390/polym6092371
Khiar A, Arof A (2010) Conductivity studies of starch-based polymer electrolytes. Ionics 16(2):123–129. https://doi.org/10.1007/s11581-009-0356-y
Gong S, Huang Y, Cao H, Lin Y, Li Y, Tang S, Wang M, Li X (2016) A green and environment-friendly gel polymer electrolyte with higher performances based on the natural matrix of lignin. J Power Sources 307:624–633. https://doi.org/10.1016/j.jpowsour.2016.01.030
Azzahari A, Mutalib NA, Rizwan M, Abouloula C, Selvanathan V, Sonsudin F, Yahya R (2018) Improved ionic conductivity in guar gum succinate–based polymer electrolyte membrane. High Perform Polym 30(8):993–1001. https://doi.org/10.1177/0954008318775790
Abouloula C, Rizwan M, Selvanathan V, Abdullah C, Hassan A, Yahya R, Oueriagli A (2018) A novel application for oil palm empty fruit bunch: extraction and modification of cellulose for solid polymer electrolyte. Ionics 24(12):3827–3836. https://doi.org/10.1007/s11581-018-2558-7
Rosa M, Medeiros E, Malmonge J, Gregorski K, Wood D, Mattoso L, Glenn G, Orts W, Imam S (2010) Cellulose nanowhiskers from coconut husk fibers: effect of preparation conditions on their thermal and morphological behavior. Carbohydr Polym 81(1):83–92. https://doi.org/10.1016/j.carbpol.2010.01.059
Singh R, Singh A (2013) Optimization of reaction conditions for preparing carboxymethyl cellulose from corn cobic agricultural waste. Waste Biomass Valorization 4(1):129–137. https://doi.org/10.1007/s12649-012-9123-9
Rasali N, Muzakir SK, Samsudin A (2017) A study on dielectric properties of the cellulose derivative-NH4Br-glycerol-based the solid polymer electrolyte system. Makara J Technol 21(2):65–69. https://doi.org/10.7454/mst.v21i2.3082
Regiani A, Machado GO, LeNest J-F, Gandini A, Pawlicka A (2001) Cellulose derivatives as solid electrolyte matrixes. Macromol Symp 175(1):45–54. https://doi.org/10.1002/1521-3900(200110)175:1<45::AID-MASY45>3.0.CO;2-M
Zainuddin N, Saadiah M, Majeed AA, Samsudin A (2018) Characterization on conduction properties of carboxymethyl cellulose/kappa carrageenan blend-based polymer electrolyte system. Int J Polym Anal Charact 23(4):321–330. https://doi.org/10.1080/1023666X.2018.1446887
Chen H (2014) Chemical composition and structure of natural lignocellulose. In: Biotechnology of lignocellulose. Springer, pp 25–71. https://doi.org/10.1007/978-94-007-6898-7_2
Thakur VK, Thakur MK, Raghavan P, Kessler MR (2014) Progress in green polymer composites from lignin for multifunctional applications: a review. J ACS Sustain Chem Eng Mining J 2(5):1072–1092. https://doi.org/10.1021/sc500087z
Samsudin A, Isa M Conductivity and transport properties study of plasticized carboxymethyl cellulose (CMC) based solid biopolymer electrolytes (SBE). In: Advanced Materials Research, 2014. Trans Tech Publ, pp 118–122. https://doi.org/10.4028/www.scientific.net/AMR.856.118
Fahma F, Iwamoto S, Hori N, Iwata T, Takemura A (2011) Effect of pre-acid-hydrolysis treatment on morphology and properties of cellulose nanowhiskers from coconut husk. Cellulose 18(2):443–450. https://doi.org/10.1007/s10570-010-9480-0
Pushpamalar V, Langford S, Ahmad M, Lim Y (2006) Optimization of reaction conditions for preparing carboxymethyl cellulose from sago waste. Carbohydr Polym 64(2):312–318. https://doi.org/10.1016/j.carbpol.2005.12.003
Cerrutti B, Souza CD, Castellan A, Ruggiero R, Frollini E (2012) Carboxymethyl lignin as stabilizing agent in aqueous ceramic suspensions. Ind Crop Prod 36(1):108–115. https://doi.org/10.1016/j.indcrop.2011.08.015
Gan L, Zhou M, Qiu X Preparation of water-soluble carboxymethylated lignin from wheat straw alkali lignin. In: Advanced Materials Research, 2012. pp 1293–1298. https://doi.org/10.4028/www.scientific.net/AMR.550-553.1293, Preparation of Water-Soluble Carboxymethylated Lignin from Wheat Straw Alkali Lignin
Jin Z, Katsumata K, Lam T, Iiyama K (2006) Covalent linkages between cellulose and lignin in cell walls of coniferous and nonconiferous woods. Biopolymers: Orig Res Biomolec 83(2):103–110. https://doi.org/10.1002/bip.20533
Thakur V, Ding G, Ma J, Lee P, Lu X (2012) Hybrid materials and polymer electrolytes for electrochromic device applications. Adv Mater 24(30):4071–4096. https://doi.org/10.1002/adma.201200213
Machado G, Ferreira H, Pawlicka A (2005) Influence of plasticizer contents on the properties of HEC-based solid polymeric electrolytes. Electrochim Acta 50(19):3827–3831. https://doi.org/10.1016/j.electacta.2005.02.041
Lee B, Oh E (2013) Effect of molecular weight and degree of substitution of a sodium-carboxymethyl cellulose binder on Li4Ti5O12 anodic performance. J Phys Chem C 117(9):4404–4409. https://doi.org/10.1021/jp311678p
Segal L, Creely J, Martin Jr AE, Conrad C (1959) An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Text Res J 29(10):786–794. https://doi.org/10.1177/004051755902901003
Kim J, Netravali A (2013) Fabrication of advanced “green” composites using potassium hydroxide (KOH) treated liquid crystalline (LC) cellulose fibers. J Mater Sci 48(11):3950–3957. https://doi.org/10.1007/s10853-013-7199-7
Park S, Baker J, Himmel M, Parilla P, Johnson D (2010) Cellulose crystallinity index: measurement techniques and their impact on interpreting cellulase performance. Biotechnol Biofuels 3(1):10. https://doi.org/10.1186/1754-6834-3-10
Lee C, Dazen K, Kafle K, Moore A, Johnson D, Park S, Kim S (2015) Correlations of apparent cellulose crystallinity determined by XRD, NMR, IR, Raman, and SFG methods. In: cellulose chemistry and properties: fibers, nanocelluloses and advanced materials. Springer, pp 115-131. https://doi.org/10.1007/12_2015_320
Wada M, Heux L, Sugiyama J (2004) Polymorphism of cellulose I family: reinvestigation of cellulose IVI. Biomacromolecules 5(4):1385–1391. https://doi.org/10.1021/bm0345357
Nishimura H, Kamiya A, Nagata T, Katahira M, Watanabe T (2018) Direct evidence for α ether linkage between lignin and carbohydrates in wood cell walls. Sci Rep 8(1):6538. https://doi.org/10.1038/s41598-018-24328-9
Yadav M, Rhee K, Jung I, Park S (2013) Eco-friendly synthesis, characterization and properties of a sodium carboxymethyl cellulose/graphene oxide nanocomposite film. Cellulose 20(2):687–698. https://doi.org/10.1007/s10570-012-9855-5
Calcagnile P, Cacciatore G, Demitri C, Montagna F, Corcione CE (2018) A feasibility study of processing polydimethylsiloxane–sodium carboxymethylcellulose composites by a low-cost fused deposition modeling 3D printer. Materials 11(9):1578. https://doi.org/10.3390/ma11091578
Doh S, Lee J, Lim D, Im J (2013) Manufacturing and analyses of wet-laid nonwoven consisting of carboxymethyl cellulose fibers. Fiber Polym 14(12):2176–2184. https://doi.org/10.1007/s12221-013-2176-y
Long L, Wang S, Xiao M, Meng Y (2016) Polymer electrolytes for lithium polymer batteries. J Mater Chem A 4(26):10038–10069. https://doi.org/10.1039/C6TA02621D
Arof A, Amirudin S, Yusof S, Noor I (2014) A method based on impedance spectroscopy to determine transport properties of polymer electrolytes. Phys Chem Chem Phys 16(5):1856–1867. https://doi.org/10.1039/C3CP53830C
Teo L, Buraidah M, Nor A, Majid S (2012) Conductivity and dielectric studies of Li2SnO3. Ionics 18(7):655–665. https://doi.org/10.1007/s11581-012-0667-2
Acknowledgements
The authors thank the financial support from Universiti Malaya through FG031-17AFR and the kind support from Dr. Izlina Binti Supa’at from Centre for Foundation Studies in Science, Universiti Malaya for the usage of the grinding machine.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 18 kb)
Rights and permissions
About this article
Cite this article
Chua, K.Y., Azzahari, A.D., Abouloula, C.N. et al. Cellulose-based polymer electrolyte derived from waste coconut husk: residual lignin as a natural plasticizer. J Polym Res 27, 115 (2020). https://doi.org/10.1007/s10965-020-02110-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-020-02110-8