Abstract
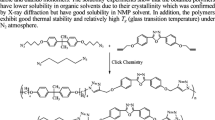
In this study, we firstly present an efficient synthetic pathway to a new Schiff base derivative (Sb) containing a thiadiazole backbone. In concomitant step, two newly designed polymers of this Schiff base derivative bearing bithiophene or fluorene subunits, abbreviated as poly[Sb-BTh] and poly[Sb-Flu], respectively, were prepared by surveying the Suzuki-Miyaura polycondensation reaction conditions. Following the completion of the synthetic steps, the appropriate structural analyses of the synthesized Schiff base and its corresponding bithiophene or fluorene bearing polymers were performed by using FT-IR, 1H-NMR, 13C-NMR, GPC, DSC, and TGA analytical techniques. Furthermore, a detailed DFT-based computational study was also performed to enlighten the conformity between the experimental and theoretical findings by investigating the fundamental physical properties of the monomer and its oligomer units (n = 1–4). The experimental studies proved that the thermal stability of the poly[Sb-BTh] was found to be better than poly[Sb-Flu], and the computational studies were found to be consistent with the experimental outcomes.










Similar content being viewed by others
References
Das P, Linert W (2016) Schiff base-derived homogeneous and heterogeneous palladium catalysts for the Suzuki–Miyaura reaction. Coord Chem Rev 311:1–23. https://doi.org/10.1016/j.ccr.2015.11.010
Ye Y-X, Liu W-L, Ye B-H (2017) A highly efficient and recyclable Pd(II) metallogel catalyst: a new scaffold for Suzuki-Miyaura coupling. Catal Commun 89:100–105. https://doi.org/10.1016/j.catcom.2016.10.017
Ben Halima T, Zhang W, Yalaoui I, Hong X, Yang YF, Houk KN, Newman SG (2017) Palladium-catalyzed Suzuki–Miyaura coupling of aryl esters. J Am Chem Soc 139:1311–1318. https://doi.org/10.1021/jacs.6b12329
Marrocchi A, Facchetti A, Lanari D, Petrucci C, Vaccaro L (2016) Current methodologies for a sustainable approach to π-conjugated organic semiconductors. Energy Environ Sci 9:763–786. https://doi.org/10.1039/C5EE03727A
Durak LJ, Payne JT, Lewis JC (2016) Late-stage diversification of biologically active molecules via Chemoenzymatic C–H functionalization. ACS Catal 6:1451–1454. https://doi.org/10.1021/acscatal.5b02558
Lennox AJJ, Lloyd-Jones GC (2014) Selection of boron reagents for Suzuki–Miyaura coupling. Chem Soc Rev 43:412–443. https://doi.org/10.1039/C3CS60197H
Isley NA, Gallou F, Lipshutz BH (2013) Transforming Suzuki–Miyaura cross-couplings of MIDA Boronates into a green technology: no organic solvents. J Am Chem Soc 135:17707–17710. https://doi.org/10.1021/ja409663q
Graham KR, Cabanetos C, Jahnke JP, Idso MN, el Labban A, Ngongang Ndjawa GO, Heumueller T, Vandewal K, Salleo A, Chmelka BF, Amassian A, Beaujuge PM, McGehee MD (2014) Importance of the donor:fullerene intermolecular arrangement for high-efficiency organic Photovoltaics. J Am Chem Soc 136:9608–9618. https://doi.org/10.1021/ja502985g
Rivnay J, Owens RM, Malliaras GG (2014) The rise of organic bioelectronics. Chem Mater 26:679–685. https://doi.org/10.1021/cm4022003
Lakhwani G, Rao A, Friend RH (2014) Bimolecular recombination in organic Photovoltaics. Annu Rev Phys Chem 65:557–581. https://doi.org/10.1146/annurev-physchem-040513-103615
Sun M, Wang W, Liang L, Yan SH, Zhou ML, Ling QD (2015) Substituent effects on direct arylation polycondensation and optical properties of alternating fluorene-thiophene copolymers. Chin J Polym Sci 33:783–791. https://doi.org/10.1007/s10118-015-1555-9
Samsonidze G, Ribeiro FJ, Cohen ML, Louie SG (2014) Quasiparticle and optical properties of polythiophene-derived polymers. Phys Rev B 90:035123. https://doi.org/10.1103/PhysRevB.90.035123
Mehmood U, Al-Ahmed A, Hussein IA (2016) Review on recent advances in polythiophene based photovoltaic devices. Renew Sust Energ Rev 57:550–561. https://doi.org/10.1016/j.rser.2015.12.177
Abdulrazzaq M, Ozkut MI, Gokce G, Ertan S, Tutuncu E, Cihaner A (2017) A low band gap polymer based on Selenophene and Benzobis (thiadiazole). Electrochim Acta 249:189–197. https://doi.org/10.1016/j.electacta.2017.08.007
Bathula C, Kalode P, Patil S, Lee SK, Belavagi NS, Khazi IA, Kang Y (2016) Synthesis and electronic properties of thiadiazole[3,4-c] pyridine copolymers for solar cell application. Tetrahedron Lett 57:998–1002. https://doi.org/10.1016/j.tetlet.2016.01.063
Qin Y, Uddin MA, Chen Y, Jang B, Zhao K, Zheng Z, Yu R, Shin TJ, Woo HY, Hou J (2016) Highly efficient fullerene-free polymer solar cells fabricated with Polythiophene derivative. Adv Mater 28:9416–9422. https://doi.org/10.1002/adma.201601803
Phung Hai TA, Sugimoto R (2016) Conjugated carbazole-thiophene copolymer: synthesis, characterization and applications. Synth Met 220:59–71. https://doi.org/10.1016/j.synthmet.2016.05.026
Hu Y, Hu D, Ming S, Duan X, Zhao F, Hou J, Xu J, Jiang F (2016) Synthesis of polyether-bridged bithiophenes and their electrochemical polymerization to electrochromic property. Electrochim Acta 189:64–73. https://doi.org/10.1016/j.electacta.2015.12.091
Sui A, Shi X, Tian H, Geng Y, Wang F (2014) Suzuki–Miyaura catalyst-transfer polycondensation with Pd(IPr)(OAc) 2 as the catalyst for the controlled synthesis of polyfluorenes and polythiophenes. Polym Chem 5:7072–7080. https://doi.org/10.1039/C4PY00917G
Naga N, Miyanaga T, Furukawa H (2014) Synthesis and optical properties of organic-inorganic hybrid semi-interpenetrating polymer network gels containing polyfluorenes. J Polym Sci Part A Polym Chem 52:973–984. https://doi.org/10.1002/pola.27077
Xiang C, Wan H, Zhu M, Chen Y, Peng J, Zhou G (2017) Dipicolylamine functionalized Polyfluorene based gel with lower critical solution temperature: preparation, characterization, and application. ACS Appl Mater Interfaces 9:8872–8879. https://doi.org/10.1021/acsami.7b00600
Muthuraj B, Mukherjee S, Patra CR, Iyer PK (2016) Amplified fluorescence from Polyfluorene nanoparticles with dual state emission and aggregation caused red shifted emission for live cell imaging and Cancer Theranostics. ACS Appl Mater Interfaces 8:32220–32229. https://doi.org/10.1021/acsami.6b11373
Li K, Liu B (2014) Polymer-encapsulated organic nanoparticles for fluorescence and photoacoustic imaging. Chem Soc Rev 43:6570–6597. https://doi.org/10.1039/C4CS00014E
Wu T-Y, Li J-L (2016) Electrochemical synthesis, optical, electrochemical and electrochromic characterizations of indene and 1,2,5-thiadiazole-based poly(2,5-dithienylpyrrole) derivatives. RSC Adv 6:15988–15998. https://doi.org/10.1039/C5RA27902J
Tong J, An L, Li J, Zhang P, Guo P, Yang C, Su Q, Wang X, Xia Y (2017) Large branched alkylthienyl bridged naphtho[1,2- c :5,6- c ′]bis[1,2,5]thiadiazole-containing low bandgap copolymers: synthesis and photovoltaic application. J Macromol Sci Part A 54:176–185. https://doi.org/10.1080/10601325.2017.1265404
Choi H, Ko S-J, Kim T, Morin PO, Walker B, Lee BH, Leclerc M, Kim JY, Heeger AJ (2015) Small-Bandgap polymer solar cells with unprecedented short-circuit current density and high fill factor. Adv Mater 27:3318–3324. https://doi.org/10.1002/adma.201501132
Chen Z, Cai P, Chen J, Liu X, Zhang L, Lan L, Peng J, Ma Y, Cao Y (2014) Low band-gap conjugated polymers with strong Interchain aggregation and very high hole mobility towards highly efficient thick-film polymer solar cells. Adv Mater 26:2586–2591. https://doi.org/10.1002/adma.201305092
Wei S, Xia J, Dell EJ, Jiang Y, Song R, Lee H, Rodenbough P, Briseno AL, Campos LM (2014) Bandgap engineering through controlled oxidation of Polythiophenes. Angew Chemie Int Ed 53:1832–1836. https://doi.org/10.1002/anie.201309398
Chen L, Wang K, Mahmoud SM, Li Y, Huang H, Huang W, Xu J, Dun C, Carroll D, Pietrangelo A (2015) Effects of replacing thiophene with 5,5-dimethylcyclopentadiene in alternating poly(phenylene), poly(3-hexylthiophene), and poly(fluorene) copolymer derivatives. Polym Chem 6:7533–7542. https://doi.org/10.1039/C5PY01247C
Miyaura N, Suzuki A (1995) Palladium-catalyzed cross-coupling reactions of Organoboron compounds. Chem Rev 95:2457–2483. https://doi.org/10.1021/cr00039a007
Frisch MJ, Trucks GW, Schlegel HB, et al (2009) Gaussian 09, revision B.01. Gaussian 09, Revis. B.01, Gaussian, Inc., Wallingford CT
Dennington R, Keith T, Millam J (2009) GaussView, Version 5. Semichem Inc., Shawnee Mission. KS
Poverenov E, Sheynin Y, Zamoshchik N, Patra A, Leitus G, Perepichka IF, Bendikov M (2012) Flat conjugated polymers combining a relatively low HOMO energy level and band gap: polyselenophenes versus polythiophenes. J Mater Chem 22:14645. https://doi.org/10.1039/c2jm31035j
Planells M, Nikolka M, Hurhangee M, Tuladhar PS, White AJP, Durrant JR, Sirringhaus H, McCulloch I (2014) The effect of thiadiazole out-backbone displacement in indacenodithiophene semiconductor polymers. J Mater Chem C 2:8789–8795. https://doi.org/10.1039/C4TC01500B
Mart H, Vilayetoglu AR (2004) Synthesis, characterization and thermal degradation of some copper (II), zinc (II) and cobalt (II) complexes with oligosalicylaldehyde. Polym Degrad Stab 83:255–258. https://doi.org/10.1016/S0141-3910(03)00270-2
Kaya İ, Koyuncu S (2003) The synthesis and characterization of oligo-N-4-aminopyridine, oligo-2-[(pyridine-4-yl-imino) methyl] phenol and its some oligomer–metal complexes. Polymer (Guildf) 44:7299–7309. https://doi.org/10.1016/j.polymer.2003.09.011
Cianga I, Ivanoiu M (2006) Synthesis of poly(Schiff-base)s by organometallic processes. Eur Polym J 42:1922–1933. https://doi.org/10.1016/j.eurpolymj.2006.03.001
Andersson MR, Thomas O, Mammo W, Svensson M, Theander M, Inganäs O (1999) Substituted polythiophenes designed for optoelectronic devices and conductors. J Mater Chem 9:1933–1940. https://doi.org/10.1039/a902859e
Kaya İ, Gökpınar M, Kamacı M (2017) Reaction conditions, photophysical, electrochemical, conductivity, and thermal properties of polyazomethines. Macromol Res 25:739–748. https://doi.org/10.1007/s13233-017-5072-2
Rasmussen S (2013) Low-Bandgap polymers. In: Encycl. Polym. Nanomater. Springer Berlin Heidelberg, Berlin, Heidelberg, pp 1–13
Kurtay G, Soganci T, Ak M, Gullu M (2016) Synthesis and computational Bandgap engineering of new 3,4-Alkylenedioxypyrrole (ADOP) derivatives and investigation of their Electrochromic properties. J Electrochem Soc 163:H896–H905. https://doi.org/10.1149/2.0131610jes
Roncali J (1997) Synthetic principles for Bandgap control in linear π-conjugated systems. Chem Rev 97:173–206. https://doi.org/10.1021/cr950257t
Roncali J (2007) Molecular engineering of the band gap of π-conjugated systems: facing technological applications. Macromol Rapid Commun 28:1761–1775. https://doi.org/10.1002/marc.200700345
Beaujuge PM, Amb CM, Reynolds JR (2010) Spectral engineering in π-conjugated polymers with Intramolecular donor−acceptor interactions. Acc Chem Res 43:1396–1407. https://doi.org/10.1021/ar100043u
Acknowledgements
Financial support from Muş Alparslan University Research Foundation (Project No: MŞÜ-14-EMF-G01) is greatly acknowledged by the authors.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 14367 kb)
Rights and permissions
About this article
Cite this article
Kaya, E., Kurtay, G. & Korkmaz, A. Combined DFT-experimental investigation and preparation of two new Thiadiazole-based Bithiophene or Fluorene containing polymers via Suzuki-Miyaura reactions. J Polym Res 27, 131 (2020). https://doi.org/10.1007/s10965-020-02086-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-020-02086-5