Abstract
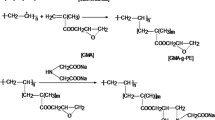
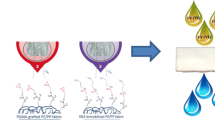
This study focuses on preparation of new adsorbent for copper by radiation induced grafting (RIG) of 2,3-epoxypropyl methacrylate (EMPA) onto PP/PE non-woven fabric (NWF) with subsequent functionalization with phosphoric acid (PA). The density of phosphoric acid incorporated onto EMPA-g-NWF was tuned by reaction parameters optimization such as PA concentration, reaction temperature, reaction time and EMPA grafting yield by using response surface method (RSM) engaging Box-Behnken design (BBD). Maximum PA density of 2.0 mmol/g−1 was estimated at optimum reaction parameter of 45.50% PA concentration, 70 °C reaction temperature, 2.76 h reaction time and 132.68% grafting yield. The deviation between optimum experimental and projected phosphoric acid density was taken into consideration, in order to verify the consistency of RSM in predicting the yield and optimizing the functionalization reaction parameters. The PA-functionalized EMPA-g-NWF was assessed with X-ray diffraction (XRD), Fourier-transform infrared (FT-IR) spectroscopy, thermogravimetric (TGA), X-ray photoelectron spectroscopy (XPS), and scanning electron microscopy-energy dispersive x-ray (SEM-EDX) to examine the crystallinity, chemical composition, thermal stability and morphology, and respectively. The preliminary study on adsorption of copper was carried out with PA-functionalized EMPA-g-NWF at different conditions such as different initial copper concentration, PA density and contact time. The maximum adsorption was obtained at pH of 4. The adsorption capacity increases as the PA density increases and maximum adsorption obtained at 2.5 mmol/g-ad of PA density. The adsorption results obtained was very promising and suggested PA-functionalized EMPA-g-NWF as a suitable candidate for copper removal.













Similar content being viewed by others
References
Demiral H, Güngör C (2016) Adsorption of copper (II) from aqueous solutions on activated carbon prepared from grape bagasse. J Clean Prod 124:103–113
Benzaoui T, Selatnia A, Djabali D (2018) Adsorption of copper (II) ions from aqueous solution using bottom ash of expired drugs incineration. Adsorption Science & Technology 36(1–2):114–129
Feng N, Guo X, Liang S (2009) Adsorption study of copper (II) by chemically modified orange peel. J Hazard Mater 164(2–3):1286–1292
Ting T, Nasef MM, Hashim K (2015) Tuning N-methyl-D-glucamine density in a new radiation grafted poly (vinyl benzyl chloride)/nylon-6 fibrous boron-selective adsorbent using the response surface method. RSC Adv 5(47):37869–37880
Kılıc M et al (2013) Adsorption of heavy metal ions from aqueous solutions by bio-char, a by-product of pyrolysis. Appl Surf Sci 283:856–862
Madrid JF, Nuesca GM, Abad LV (2013) Gamma radiation-induced grafting of glycidyl methacrylate (GMA) onto water hyacinth fibers. Radiat Phys Chem 85:182–188
Madrid JF, Nuesca GM, Abad LV (2014) Amine functionalized radiation-induced grafted water hyacinth fibers for Pb 2+, cu 2+ and Cr 3+ uptake. Radiat Phys Chem 97:246–252
Li K, Zheng Z, Li Y (2010) Characterization and lead adsorption properties of activated carbons prepared from cotton stalk by one-step H3PO4 activation. J Hazard Mater 181(1–3):440–447
Nasef MM, Nallappan M, Ujang Z (2014) Polymer-based chelating adsorbents for the selective removal of boron from water and wastewater: a review. React Funct Polym 85:54–68
Dispenza, C., S. Alessi, and J. Spadaro, Radiation processing of polymers in aqueous media. Applications of Ionizing Radiation in Materials Processing. Warszawa: INCT, 2017: p. 291–326
Tamada M, Seko N, Yoshii F (2004) Application of radiation-graft material for metal adsorbent and crosslinked natural polymer for healthcare product. Radiat Phys Chem 71(1–2):223–227
Hokkanen S et al (2014) Adsorption of Ni (II), cu (II) and cd (II) from aqueous solutions by amino modified nanostructured microfibrillated cellulose. Cellulose 21(3):1471–1487
Dong T et al (2017) Effect of immobilized amine density on cadmium (II) adsorption capacities for ethanediamine-modified magnetic poly-(glycidyl methacrylate) microspheres. J Magn Magn Mater 427:289–295
Samiey B, Cheng C-H, Wu J (2014) Organic-inorganic hybrid polymers as adsorbents for removal of heavy metal ions from solutions: a review. Materials 7(2):673–726
Selambakkannu S et al (2018) A kinetic and mechanistic study of adsorptive removal of metal ions by imidazole-functionalized polymer graft banana fiber. Radiat Phys Chem 153:58–69
Mourabet M et al (2012) Removal of fluoride from aqueous solution by adsorption on Apatitic tricalcium phosphate using box–Behnken design and desirability function. Appl Surf Sci 258(10):4402–4410
Petrovich J (2015) FTIR and DSC of polymer films used for packaging: LLDPE. PP and PVDC, SHAPE American High School
Li R et al (2018) Phosphate-based ultrahigh molecular weight polyethylene fibers for efficient removal of uranium from carbonate solution containing fluoride ions. Molecules 23(6):1245
Wu G, Lin S, Yang C (2006) Preparation and characterization of high ionic conducting alkaline non-woven membranes by sulfonation. J Membr Sci 284(1–2):120–127
Vassal N, Salmon E, Fauvarque J-F (2000) Electrochemical properties of an alkaline solid polymer electrolyte based on P (ECH-co-EO). Electrochim Acta 45(8–9):1527–1532
Montes-Atenas G, Schroeder SL (2015) Sustainable natural adsorbents for heavy metal removal from wastewater: lead sorption on pine bark (Pinus radiata D. Don). Surf Interface Anal 47(10):996–1000
Ren Z, Xu X, Wang X, Gao B, Yue Q, Song W, Zhang L, Wang H (2016) FTIR, Raman, and XPS analysis during phosphate, nitrate and Cr (VI) removal by amine cross-linking biosorbent. J Colloid Interface Sci 468:313–323
Deng S, Ting YP (2005) Polyethylenimine-modified fungal biomass as a high-capacity biosorbent for Cr (VI) anions: sorption capacity and uptake mechanisms. Environmental science & technology 39(21):8490–8496
Esposito A et al (2001) Biosorption of heavy metals by Sphaerotilus natans: an equilibrium study at different pH and biomass concentrations. Hydrometallurgy 60(2):129–141
El Ghali A, Baouab MHV, Roudesli MS (2011) Preparation, characterization and application of a [copper (II)/ethylenediamine–cotton] complex for the removal of AB25 from aqueous solution in a laboratory scale column. Chem Eng J 174(1):18–26
Acknowledgements
Nor Azillah Fatimah concede the sponsorship of the study by Malaysia Nuclear Agency.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Othman, N.A.F., Selambakkannu, S., Ting, T.M. et al. Integration of phosphoric acid onto radiation grafted poly (2,3-epoxypropyl methacrylate) -PP/PE non-woven fabrics aimed copper adsorbent via response surface method. J Polym Res 26, 283 (2019). https://doi.org/10.1007/s10965-019-1963-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-019-1963-6