Abstract
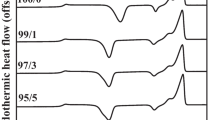
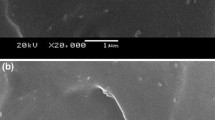
In this work, the poly(butylene succinate) (PBSu)/orotic acid (OA) composites were thoroughly investigated. By the observation of scanning electron microscopy, we found that OA uniformly dispersed in PBSu matrix. For the results of isothermal crystallization, the isothermal crystallization peak shifts to the shorter time frame with increasing the OA content, suggesting the promotion of crystallization rate by adding OA in PBSu. Isothermal crystallization kinetics were analyzed using the Avrami equation. The rate constant (k) increases when the amount of OA is increased. This result confirmed that the crystallization rate was increased with increasing the OA amount. We also discussed the non-isothermal crystallization of the PBSu/OA composites. It shows that the crystallization peak of PBSu moves to the higher temperature region after adding OA, suggesting the enhancement of non-isothermal crystallization caused by OA. The analysis of the Mo model demonstrates that with the presence of OA, the F(T) value is decreased. This can also indicate that OA would promote PBSu’s crystallization rate of non-isothermal crystallization. The results in this work demonstrate that OA can significantly increase PBSu’s crystallization rate. OA can be an effective nucleation agent of PBSu.









Similar content being viewed by others
References
Hikima Y, Morikawa J, Hashimoto T (2013) Wavenumber dependence of FT-IR image of molecular orientation in banded spherulites of poly(3-hydroxybutyrate) and poly(l-lactic acid). Macromolecules 46:1582–1590
Barrett JS, Abdala AA, Srienc F (2014) Poly (hydroxyalkanoate) elastomers and their graphene nanocomposites. Macromolecules 47:3926–3941
Wang X, Zhuang Y, Dong L (2012) Study of carbon black-filled poly(butylene succinate)/polylactide blend. J Appl Polym Sci 126:1876–1884
Ojijo V, Sinha Ray S, Sadiku R (2012) Role of specific interfacial area in controlling properties of immiscible blends of biodegradable polylactide and poly[(butylene succinate)-co-adipate]. ACS Appl Mater Interfaces 4:6690–6701
Woo EM, Yen KC, Yeh YT, Wang LY (2018) Biomimetically structured lamellae assembly in periodic banding of poly(ethylene adipate) crystals. Macromolecules 51:3845–3854
Huang CF, Chen WH, Aimi J, Huang YS, Venkatesan S, Chiang YW, Huang SH, Kuo SW, Chen T (2019) Synthesis of well-defined PCL-b-PnBA-b-PMMA ABC-type triblock copolymers: toward the construction of nanostructures in epoxy thermosets. Polym Chem 9:5644–5654
Yang CT, Lee L-T, Wu TY (2018) Isothermal and nonisothermal crystallization kinetics of poly(ε-caprolactone) blended with a novel ionic liquid, 1-ethyl-3-propylimidazolium bis(trifluoromethanesulfonyl)imide. Polymers 10. https://doi.org/10.3390/polym10050543
Lee L-T, Yang CT (2016) Investigations on green blends comprising biodegradable polymer and ionic liquid. Polymers 8. https://doi.org/10.3390/polym8120444
Su HH, Chen HL, Díaz A, Casas MT, Puiggalí J, Hoskins JN, Grayson SM, Pérez RA, Müller AJ (2013) New insights on the crystallization and melting of cyclic PCL chains on the basis of a modified Thomson-Gibbs equation. Polymer 54:846–859
Jacquel N, Freyermouth F, Fenouillot F, Rousseau A, Pascault JP, Fuertes P, Saint-Loup R (2011) Synthesis and properties of poly (butylene succinate): efficiency of different transesterification catalysts. J Polym Sci A Polym Chem 49:5301–5312
Vroman I, Tighzert L (2009) Biodegradable polymers. Materials 2. https://doi.org/10.3390/ma2020307
Nerantzaki M, Koliakou I, Kaloyianni MG, Koumentakou I, Siska E, Diamanti E, Karakassides MA, Boccaccini AR, Bikiaris DN (2017) A biomimetic approach for enhancing adhesion and osteogenic differentiation of adipose-derived stem cells on poly(butylene succinate) composites with bioactive ceramics and glasses. Eur Polym J 87:159–173
Wu Z, Zheng K, Zhang J, Tang T, Guo H, Boccaccini AR, Wei J (2016) Effects of magnesium silicate on the mechanical properties, biocompatibility, bioactivity, degradability, and osteogenesis of poly(butylene succinate)-based composite scaffolds for bone repair. J Mater Chem B 4:7974–7988
Gao C, Ren X, Zhang J, Wang B, Liu Y, Wu Y (2018) Effect of magnesium hydroxide sulfate hydrate whisker on non-isothermal crystallization kinetics of poly(butylene succinate). Thermochim Acta 663:9–18
Vassiliou AA, Chrissafis K, Bikiaris DN (2009) In situ prepared PBSu/SiO2 nanocomposites. Study of thermal degradation mechanism. Thermochim Acta 495:120–128
Ye HM, Tang YR, Xu J, Guo BH (2013) Role of poly(butylene fumarate) on crystallization behavior of poly(butylene succinate). Ind Eng Chem Res 52:10682–10689
Qi Z, Ye H, Xu J, Peng J, Chen J, Guo B (2013) Synthesis and characterizations of attapulgite reinforced branched poly(butylene succinate) nanocomposites. Colloid Surf A-Physicochem Eng Asp 436:26–33
Du XC, Xu XL, Liu XH, Yang JH, Wang Y, Gao XL (2016) Graphene oxide induced crystallization and hydrolytic degradation of poly(butylene succinate). Polym Degrad Stab 123:94–104
Song L, Qiu Z (2009) Crystallization behavior and thermal property of biodegradable poly(butylene succinate)/functional multi-walled carbon nanotubes nanocomposite. Polym Degrad Stab 94:632–637
Chang L, Woo EM (2012) Crystallization of poly(3-hydroxybutyrate) with stereocomplexed polylactide as biodegradable nucleation agent. Polym Eng Sci 52:1413–1419
Pan P, Yang J, Shan G, Bao Y, Weng Z, Inoue Y (2012) Nucleation effects of nucleobases on the crystallization kinetics of poly(l-lactide). Macromol Mater Eng 297:670–679
Li Z, Kong J, Han L, Zhang H, Dong L (2018) Effect of crystallinity on the thermal conductivity of poly(3-hydroxybutyrate)/BN composites. Polym Bull 75:1651–1666
Chen J, Xu C, Wu D, Pan K, Qian A, Sha Y, Wang L, Tong W (2015) Insights into the nucleation role of cellulose crystals during crystallization of poly(β-hydroxybutyrate). Carbohydr Polym 134:508–515
Lanfranconi M, Alvarez VA, Luduena LN (2016) Isothermal crystallization of polycaprolactone/modified clay biodegradable nanocomposites. J Therm Anal 126:1273–1280
Refaa Z, Boutaous MH, Xin S, Fulchiron R (2017) Synergistic effects of shear flow and nucleating agents on the crystallization mechanisms of poly(lactic acid). J Polym Res 24. https://doi.org/10.1007/s10965-016-1179-y
Richards SM, Conyers RA, Fisher JL, Rosenfeldt FL (1997) Cardioprotection by orotic acid: metabolism and mechanism of action. J Mol Cell Cardiol 29:3239–3250
Kim YK, Choi MJ, Oh TY, Yu KS, Lee S (2017) A comparative pharmacokinetic and tolerability analysis of the novel orotic acid salt form of tenofovir disoproxil and the fumaric acid salt form in healthy subjects. Drug Des Dev Ther 11:3171–3177
Hong ES, Kim EK, Kang SM, Khang AR, Choi SH, Park KS, Jang HC, Lim S (2014) Effect of carnitine-orotate complex on glucose metabolism and fatty liver: a double-blind, placebo-controlled study. J Gastroenterol Hepatol 29:1449–1457
Stepura OB, Martynow AI (2009) Magnesium orotate in severe congestive heart failure (MACH). Int J Cardiol 134:145–147
Torshin IY, Gromova OA, Kalacheva AG, Oshchepkova EV, Martynov AI (2015) Meta-analysis of clinical trials of cardiovascular effects of magnesium orotate. Ter Arkh 87:88–97
Jacquel N, Tajima K, Nakamura N, Miyagawa T, Pan P, Inoue Y (2009) Effect of orotic acid as a nucleating agent on the crystallization of bacterial poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) copolymers. J Appl Polym Sci 114:1287–1294
Jacquel N, Tajima K, Nakamura N, Kawachi H, Pan P, Inoue Y (2010) Nucleation mechanism of polyhydroxybutyrate and poly(hydroxybutyrate-co-hydroxyhexanoate) crystallized by orotic acid as a nucleating agent. J Appl Polym Sci 115:709–715
Tsui A, Frank CW (2014) Comparison of anhydrous and monohydrated forms of orotic acid as crystal nucleating agents for poly(3-hydroxybutyrate-co-3-hydroxyvalerate). Polymer 55:6364–6372
Qiu Z, Li Z (2011) Effect of orotic acid on the crystallization kinetics and morphology of biodegradable poly(L-lactide) as an efficient nucleating agent. Ind Eng Chem Res 50:12299–12303
Yang J, Chen Y, Hua L, Liang R, Zhu D (2016) Crystallization behavior and polymorphism of poly(1,4-butylene adipate): effect of anhydrous orotic acid as nucleating agent. J Appl Polym Sci 133. https://doi.org/10.1002/APP.42957
Hafsia KB, Ponçot M, Chapron D, Royaud I, Dahoun A, Bourson P (2016) A novel approach to study the isothermal and non-isothermal crystallization kinetics of poly(ethylene terephthalate) by Raman spectroscopy. J Polym Res 23. https://doi.org/10.1007/s10965-016-0984-7
Shi X, Jing Z, Zhang G (2018) Influence of PLA stereocomplex crystals and thermal treatment temperature on the rheology and crystallization behavior of asymmetric poly(L-lactide)/poly(D-lactide) blends. J Polym Res 25. https://doi.org/10.1007/s10965-018-1467-9
Liang Z, Pan P, Zhu B, Dong T, Inoue Y (2010) Mechanical and thermal properties of poly(butylene succinate)/plant fiber biodegradable composite. J Appl Polym Sci 115:3559–3567
Liu X, Wu Q (2002) Non-isothermal crystallization behaviors of polyamide 6/clay nanocomposites. Eur Polym J 38:1383–1389
Auliawan A, Woo EM (2012) Crystallization kinetics and degradation of nanocomposites based on ternary blend of poly(L-lactic acid), poly(methyl methacrylate), and poly(ethylene oxide) with two different organoclays. J Appl Polym Sci 125. https://doi.org/10.1002/app.36761
Yoo ES, Im SS (1999) Melting behavior of poly(butylene succinate) during heating scan by DSC. J Polym Sci Pt B-Polym Phys 37:1357–1366
Yang J, Pan P, Hua L, Xie Y, Dong T, Zhu B, Inoue Y, Feng X (2011) Fractionated crystallization, polymorphic crystalline structure, and spherulite morphology of poly(butylene adipate) in its miscible blend with poly (butylene succinate). Polymer 52:3460–3468
Acknowledgements
The research grants of MOST 107-2221-E-035-030- and MOST 108-2221-E-035-062- from Taiwan’s Ministry of Science and Technology (MOST) are appreciated.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lee, LT., Hsu, CY. & Hung, SP. Promoted crystallization kinetics of biodegradable poly(butylene succinate) by a nucleation agent of green chemical. J Polym Res 26, 255 (2019). https://doi.org/10.1007/s10965-019-1929-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-019-1929-8