Abstract
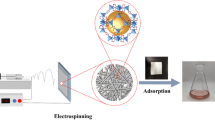
In this work, polyacrylonitrile (PAN) nanofibers were utilized to prepare metal-organic framework (MOF) nanofibrous mats. Herein, 2-methylimidazole (2-MI) was mixed in polymeric solution for electrospinning. Subsequently, zeolitic imidazolate framework (ZIF-8) nanocrystals were growth on the surface of PAN nanofibers through post-treatment. To confirm the structures, ZIF-8/PAN nanofibrous mats were investigated by FTIR, XRD, SEM, and TGA measurements. The results presented uniform ZIF-8/PAN nanofibrous mats were fabricated. Further, ZIF-8/PAN nanofibrous mats were employed for dye adsorption using methylene blue (MB) and malachite green (MG) as models. The optimum dye-adsorption conditions were investigated. The results shown the optimum concentration, temperature and pH value of for MB adsorption were 50 mg/L, 30 °C and pH = 11; for MG absorption were 300 mg/L, 30 °C and pH = 5, respectively. Meanwhile, the results displayed prepared ZIF-8/PAN nanofibrous mats presented well adsorption capacity to MG with a quick adsorption rate, which reached to 1531.94 mg/g. Moreover, MB and MG adsorption isotherms of ZIF-8/PAN nanofibrous mats were best fitted by the Langmuir isotherm model, and the results presented pseudo second-order kinetic equation was more consistent with the MB and MG adsorption processes of the adsorbent. Additionally, ZIF-8/PAN nanofibrous mats are successfully degradation MB under xenon light irradiation.

Graphical abstract










Similar content being viewed by others
References
Arshadi M, Faraji AR, Amiri MJ, Mehravar M, Gil A (2015) Removal of methyl orange on modified ostrich bone waste--a novel organic-inorganic biocomposite. J Colloid Interface Sci 446:11–23
Feng Y, Li Y, Xu MY, Liu SC, Yao JF (2016) Fast adsorption of methyl blue on zeolitic imidazolate framework-8 and its adsorption mechanism. RSC Adv 6:109608–109612
Tabaraki R, Nateghi A (2018) Removal of methylene blue, malachite green and rhodamine B in a ternary system by pistachio hull; application of wavelet neural network modeling and doehlert design. Anal Bioanal Chem 5:143–157
Zhu ZG, Wu P, Liu GJ, He XF, Qi BY, Zeng GF, Wang W, Sun YH, Cui FY (2017) Ultrahigh adsorption capacity of anionic dyes with sharp selectivity through the cationic charged hybrid nanofibrous membranes. Chem Eng J 313:957–966
Zhu ZG, Li GH, Zeng GF, Chen XQ, Hu D, Zhang YF, Sun YF (2015) Fast capture of methyl-dyes over hierarchical amino-Co0.3Ni0.7Fe2O4@SiO2 nanofibrous membranes. J Mater Chem A 3:22000–22004
Wang CC, Zhang J, Wang P, Wang H, Yang H (2015) Adsorption of methylene blue and methyl violet by camellia seed powder: kinetic and thermodynamic studies. Desalin Water Treat 53:3681–3690
Du ZF, Guo RH, Lan JW, Jiang SX, Lin SJ, Cheng C, Zhao LD (2017) Bismuth tungstate coating on polyester fabric modified with dopamine for photocatalytic property under visible light irradiation. Surf Coat Technol 319:219–229
Robinson T, Chandran B, Nigam P (2002) Removal of dyes from a synthetic textile dye effluent by biosorption on apple pomace and wheat straw. Water Res 36:2824–2830
Annadural G, Juang RS, Lee DJ (2002) Use of cellulose-based wastes for adsorption of dyes from aqueous solutions. J Hazard Mater 92:263–274
Huo SH, Yan XP (2012) Metal–organic framework MIL-100(Fe) for the adsorption of malachite green from aqueous solution. J Mater Chem 22:7449–7455
Zhu XS, Jiang XS, Cheng S, Wang K, Mao SL, Fan LJ (2010) Preparation of high strength ultrafine polyvinyl chloride fibrous membrane and its adsorption of cationic dye. J Polym Res 17:769–777
Eddaoudi M, Kim J, Rosi N, Vodak D, Wachter J, O'Keeffe M, Yaghi OM (2002) Systematic design of pore size and functionality in isoreticular MOFs and their application in methane storage. Science 295:469–472
Kim J, Chen BL, Reineke TM, Li HL, Eddaoudi M, Moler DB, O'Keeffe M, Yaghi OM (2001) Assembly of metal-organic frameworks from large organic and inorganic secondary building units: new examples and simplifying principles for complex structures. J Am Chem Soc 123:8239–8247
Salles F, Jobic H, Maurin G, Koza MM, Llewellyn PL, Devic T, Serre C, Ferey G (2008) Experimental evidence supported by simulations of a very high H2 diffusion in metal organic framework materials. Phys Rev Left 100:24
Feng Y, Li Y, Xu MY, Liu SC, Yao JF (2016) Fast adsorption of methyl blue on zeolitic imidazolate framework-8 and its adsorption mechanism. RSC Adv 6:109808–109612
Haque E, Jun JW, Jhung SH (2011) Adsorptive removal of methyl orange and methylene blue from aqueous solution with a metal-organic framework material, iron terephthalate (MOF-235). J Hazard Mater 185:507–511
Zheng J, Cheng C, Fang WJ, Chen C, Yan RW, Huai HX, Wang CC (2014) Surfactant-free synthesis of a Fe3O4@ZIF-8 core–shell heterostructure for adsorption of methylene blue. Cryst Eng Comm 16:3960–3964
Abdi J, Vossoughi M, Mahmoodi NM, Alemzadeh I (2017) Synthesis of metal-organic framework hybrid nanocomposites based on GO and CNT with high adsorption capacity for dye removal. Chem Eng J 326:1145–1158
Jing HP, Wang CC, Zhang YW, Wanga P, Li R (2014) Photocatalytic degradation of methylene blue in ZIF-8. RSC Adv 4:54454–54462
Nam CW, Kim YH, Ko SW (1999) Modification of plyacrylonitrile (PAN) fiber by blending with N-(2-Hydroxy)propyl-3-trimethylammonium chitosan chloride. J Appl Polym Sci 74:2258–2265
Yin X, Cheng H, Wang X, Yao Y (1998) Morphology and properties of hollow-fiber membrane made by PAN mixing with small amount of PVDF. J Membr Sci 146:179–184
Cravillon J, Munzer S, Lohmeier SJ, Feldhoff A, Huber K, Wiebcke M (2009) Rapid room-temperature synthesis and characterization of nanocrystals of a prototypical Zeolitic Imidazolate framework. Chem Mater 21:1410–1412
Hu Y, Kazemian H, Rohani S, Huang Y, Song Y (2011) In situ high pressure study of ZIF-8 by FTIR spectroscopy. Chem Commun 47:12694–12696
Lin KYA, Chen YC, Phattarapattamawong S (2016) Efficient demulsification of oil-in-water emulsions using a zeolitic imidazolate framework: adsorptive removal of oil droplets from water. J Colloid Interface Sci 478:97–106
Liao SM, Du QS, Meng JZ, Pang ZW, Huang RB (2013) The multiple roles ofhistidine in protein interactions. Chem Cent J:7–44
Bulut Y, Aydin H (2006) A kinetics and thermodynamics study of methylene blue adsorption on wheat shells. Desalin 194:259–267
Reddad Z, Gerente C, Andres AY, Cloirec PL (2002) Adsorption of several metal ions onto a low-cost biosorbent: kinetic and equilibrium studies. Environ Sci Technol 36:2067–2073
Zhao MF, Tang ZB, Liu P (2008) Removal of methylene blue from aqueous solution withsilica nano-sheets derived from vermiculite. J Hazard Mater 158:43–51
Dai HJ, Huang Y, Huang HH (2018) Eco-friendly polyvinyl alcohol/carboxymethyl cellulose hydrogels reinforced with graphene oxide and bentonite for enhanced adsorption of methylene blue. Carbohydr Polym 185:1–11
Yu B, Zhang Y, Shukla A, Shukla SS, Dorris KL (2011) The removal of heavy metal from aqueous solutions bysawdust adsorptionremoval of copper. Hazard Mater 80:33–42
Nuengmatcha P, Mahachai R, Chanthai S (2014) Thermodynamic and kinetic study of the intrinsic adsorption capacity of graphene oxide for malachite green removal from aqueous solution. Orient J Chem 30:1463–1474
Bello OS, Ahmad MA (2011) Coconut (Cocos nucifera) shell based activated carbon for the removal of Malachite Green Dye from aqueous solutions. Sep Sci Technol 47:903–912
Tian Y, Liu P, Wang X, Lin H (2011) Adsorption of malachite green from aqueous solutions onto ordered mesoporous carbons. Chem Eng J 171:1263–1269
Abdi J, Mahmoodi NM, Vossoughi M, Alemzadeh I (2019) Synthesis of magnetic metal-organic framework nanocomposite (ZIF-8@SiO2@MnFe2O4) as a novel adsorbent for selective dye removal from multicomponent systems. Microporous Mesoporous Mater 273:177–188
Ashrafi M, Bagherian G, Chamjangali MA, Goudarzi N (2018) Application of artificial neural network and random forest methods for modeling simultaneous adsorption of safranin-O and methyl violet dyes onto modified pine cone powder. Anal Bioanal Chem Res 109:90–103
Qian WC, Luo XP, Wang X, Guo M, Li B (2018) Removal of methylene blue from aqueous solution by modified bamboo hydrochar. Ecotox Environ Safe 157:300–306
Acknowledgements
This work was financially supported by “the Fundamental Research Funds for the Central Universities”(No: YJ201823).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhan, Y., Guan, X., Ren, E. et al. Fabrication of zeolitic imidazolate framework-8 functional polyacrylonitrile nanofibrous mats for dye removal. J Polym Res 26, 145 (2019). https://doi.org/10.1007/s10965-019-1806-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-019-1806-5