Abstract
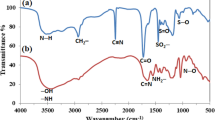
In this report, we intended to synthesize chelating grafted copolymer of gum ghatti with acrylonitrile (Gg-g-An) by gamma irradiation as proficient and influential adsorbent for removal of uranyl ions. These grafted and modified copolymers were characterized using different techniques viz. FTIR, Elemental analysis, TGA and FESEM. Maximum grafting was accomplished with 5% gum ghatti solution and 1:3 ratio of acrylonitrile to backbone at 25 kGy total radiation dose. Surface area of grafted copolymer was calculated by BET analysis. Adsorption experiments reveal the adsorption capacity was effectively influenced at pH 6 with maximum adsorption 94% which substantiated Gg-g-AO surface engross excellent potential as chelating agent for adsorption of uranyl ions. Uptake of uranyl ions by Gg-g-AO was confirmed using spectroscopic and EDX analysis. The result showed that the pseudo second order reaction and Langmuir adsorption isotherms had remarkable conformity through linear fit statistics with r2 0.998 and 0.978 respectively. Furthermore, the thermodynamic parameters illustrated that the uranyl ions adsorption process by Gg-g-AO was endothermic and spontaneous. Desorption studies of Gg-g-AO confirmed the reusability up to three cycles in 0.1 M HCl. This study substantiated that the synthesized Gg-g-AO has impending use in environmental remediation.













Similar content being viewed by others
Abbreviations
- An:
-
Acrylonitrile
- AO:
-
Amidoximation
- DMF:
-
Dimethyl formamide
- FTIR:
-
Fourier Transform Infrared Spectroscopy
- TGA:
-
Thermogravimetric analysis
- BET:
-
Brunauer–Emmett–Teller
- FESEM:
-
Field Emission Scanning Electron Microscopy
- EDX:
-
Energy dispersive X-ray Analysis
- Gg:
-
Gum ghatti
- Gg-g-An:
-
Gum ghatti grafted acrylonitrile
- Gg-g-AO:
-
Amidoximated Gum ghatti grafted acrylonitrile
- %GE:
-
Percent grafting efficiency
- %GY:
-
Percent grafting yield
- %C:
-
Percent conversion
- %H:
-
Percent homopolymer
References
Kalin M, Wheeler WN, Meinrath G (2004) The removal of uranium from mining waste water using algal/microbial biomass. J Environ Radioact 78:151–177. https://doi.org/10.1016/j.jenvrad.2004.05.002
Liu X, Cheng C, Xiao C, Shao D, Xu Z, Wang J, Hu S, Li X, Wang W (2017) Polyaniline (PANI) modified bentonite by plasma technique for U(VI) removal from aqueous solution. Appl Surf Sci 411:331–337. https://doi.org/10.1016/j.apsusc.2017.03.095
Bhattacharya A, Misra BN (2004) Grafting: a versatile means to modify polymers techniques, factors and applications. Prog Polym Sci 29:767–814. https://doi.org/10.1016/j.progpolymsci.2004.05.002
Wojnárovits L, Földváry CM, Takács E (2010) Radiation-induced grafting of cellulose for adsorption of hazardous water pollutants: a review. Radiat Phys Chem 79:848–862. https://doi.org/10.1016/j.radphyschem.2010.02.006
Behari K, Pandey PK, Kumar R, Taunk K (2001) Graft copolymerization of acrylamide onto xanthan gum. Carbohydr Polym 46:185–189. https://doi.org/10.1016/S0144-8617(00)00291-5
Sharma K, Kaith BS, Kumar VV, Kumar V, Som S, Kalia S, Swart HC (2013) Synthesis and properties of poly(acrylamide-aniline)-grafted gum ghatti based nanospikes. RSC Adv 3:25830–25839. https://doi.org/10.1039/c3ra44809f
Kumar A, Singh K, Ahuja M (2009) Xanthan-g-poly(acrylamide): microwave-assisted synthesis, characterization and in vitro release behavior. Carbohydr Polym 76:261–267. https://doi.org/10.1016/j.carbpol.2008.10.014
Deshmukh AS, Setty CM, Badiger AM, Muralikrishna KS (2011) Gum ghatti: a promising polysaccharide for pharmaceutical applications. Carbohydr Polym 87:980–986. https://doi.org/10.1016/j.carbpol.2011.08.099
Pekel N, Sahiner N, Guven O (2001) Use of amidoximated acrylonitrile/N-vinyl 2-pyrrolidone interpenetrating polymer networks for uranyl ion adsorption from aqueous systems. J Appl Polym Sci 81:2324–2329. https://doi.org/10.1002/app.1673
Kiatkamjornwong S, Chvajarernpun J, Nakason C (1993) Modification on liquid retention property of cassava starch by radiation grafting with acrylonitrile. I. Effect of γ-irradiation on grafting parameters. Radiat Phys Chem 42:47–52. https://doi.org/10.1016/0969-806X(93)90200-E
Singh V, Tiwari A, Sanghi R (2005) Studies on K2S2O8/ascorbic acid initiated synthesis of Ipomoea dasysperma seed gum-g-poly(acrylonitrile): a potential industrial gum. J Appl Polym Sci 98:1652–1662. https://doi.org/10.1002/app.22333
Xu C, Wang J, Yang T, Chen X, Liu X, Ding X (2015) Adsorption of uranium by amidoximated chitosan-grafted polyacrylonitrile, using response surface methodology. Carbohydr Polym 121:79–85. https://doi.org/10.1016/j.carbpol.2014.12.024
Savvin SB (1961) Analytical use of arsenazo III: determination of thorium, zirconium, uranium and rare earth elements. Talanta 8:673–685. https://doi.org/10.1016/0039-9140(61)80164-1
Liu X, Liu H, Ma H, Cao C, Yu M, Wang Z, Deng B, Wang M, Li J (2012) Adsorption of the uranyl ions on an Amidoxime-based polyethylene nonwoven fabric prepared by Preirradiation-induced emulsion graft polymerization. Ind Eng Chem Res 51:15089–15095. https://doi.org/10.1021/ie301965g
Singh V, Kumari PL, Tiwari A, Pandey S (2010) Alumina-supported microwave synthesis of Cassia marginata seed gum-graft-polyacrylamide. J Appl Polym Sci 117:3630–3638. https://doi.org/10.1002/app.32273
Spinks JWT, Woods RJ (1990) An introduction to radiation chemistry. John Wiley and Sons, New York
Woods RJ, Pikaev AK (1994) Applied radiation chemistry: radiation processing. John Wiley and Sons Inc, New York
Mittal H, Fosso-Kankeu E, Mishra SB, Mishra AK (2013) Biosorption potential of gum ghatti-g-poly(acrylic acid) and susceptibility to biodegradation by B. subtilis. Int J Biol Macromol 62:370–378. https://doi.org/10.1016/j.ijbiomac.2013.09.023
Zahri NAM, Jamil SNAM, Abdullah LC et al (2015) Improved method for preparation of amidoxime modified poly(acrylonitrile-co-acrylic acid): characterizations and adsorption case study. Polymers (Basel) 7:1205–1220. https://doi.org/10.3390/polym7071205
Mulani K, Patil V, Chavan N, Donde K (2019) Adsorptive removal of chromium (VI) using spherical resorcinol-formaldehyde beads prepared by inverse suspention polymerization. J Polym Res 21:1–11. https://doi.org/10.1007/s10965-091-1705-9
Mittal H, Kaith BS, Jindal R (2010) Microwave radiation induced synthesis of gum ghatti and acrylamide based crosslinked network and evaluation of its thermal and electrical behavior. Der Chem Sin 22:59–69
Rani P, Sen G, Mishra S, Jha U (2012) Microwave assisted synthesis of polyacrylamide grafted gum ghatti and its application as flocculant. Carbohydr Polym 89:275–281. https://doi.org/10.1016/j.carbpol.2012.03.009
Yu H, Yang S, Ruan H, Shen J, derBruggen BV (2015) Recovery of uranium ions from simulated seawater with palygorskite/amidoximepolyacrylonitrile composite. Appl Clay Sci 111:67–75. https://doi.org/10.1016/j.clay.2015.01.035
Sen TK, Gomez D (2011) Adsorption of zinc (Zn2+) from aqueous solution on natural bentonite. Desalination 267:286–294. https://doi.org/10.1016/j.desal.2010.09.041
Hritcu D, Humelnicu D, Dodi G, Popa MI (2012) Magnetic chitosan composite particles: evaluation of thorium and uranyl ion adsorption from aqueous solutions. Carbohydr Polym 87:1185–1191. https://doi.org/10.1016/j.carbpol.2011.08.095
Lagergren S (1898) About the theory of so-called adsorption of soluble substances. K Sven Vetensk Akad Handl 24:1–39
Ho YS, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34:451–465. https://doi.org/10.1016/S0032-9592(98)00112-5
Weber WJ, Morris JC (1963) Kinetics of adsorption on carbon from solution. J Sanit Eng Div 89(2):31–60
Boyd GE, Adamson AW, Myers LS (1947) The exchange adsorption of ions from aqueous solutions by organic zeolites (II) kinetics. J Am Chem Soc 69:2836–2848. https://doi.org/10.1021/ja01203a066
Langmuir (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. platinum J Am Chem Soc 40(9):1361–1403. https://doi.org/10.1021/ja02242a004
Freundlich FMF (1906) Über die adsorption in Lösungen. Z Phys Chem 57:385–470. https://doi.org/10.1515/zpch-1907-5723
Lim LBL, Priyantha N, Tennakoon DTB, Chieng HI, Dahri MK, Suklueng M (2014) Breadnut peel as a highly effective low-cost biosorbent for methylene blue: equilibrium, thermodynamic and kinetic studies. Arab J Chem 10:S3216–S3228. https://doi.org/10.1016/j.arabjc.2013.12.018
Abdi S, Nasiri M, Mesbahi A, Khani MH (2017) Investigation of uranium (VI) adsorption by polypyrrole. J Hazard Mater 332:132–139. https://doi.org/10.1016/j.jhazmat.2017.01.013
Yang L, Bi L, Lei Z, Miao Y et al (2018) Preparation of amidoximated functionalized β- Cyclodextrin-graft-(maleic anhydride-co-Acryloniytile) copolymer and evaluation of the adsorption and regeneration properties of uranium. Polymers 236:1–18
Gok C, Aytas S (2009) Biosorption of uranium(VI) from aqueous solution using calcium alginate beads. J Hazard Mater 168:369–375. https://doi.org/10.1016/j.jhazmat.2009.02.063
Ding DX, Liu XT, Hu N, Li GY, Wang YD (2012) Removal and recovery of uranium from aqueous solution by tea waste. J Radioanal Nucl Chem 293:735–741. https://doi.org/10.1007/s10967-012-1866-z
Monier M, Abdel-Latif DA, Mohammed HA (2015) Synthesis and characterization of uranyl ion-imprinted microspheres based on amidoximated modified alginate. Int J Biol Macromol 75:354–363. https://doi.org/10.1016/j.ijbiomac.2014.12.001
Acknowledgments
The authors thank Department of Chemistry, SPPU for providing laboratory amenities. Authors are also thankful to SAIF, IIT Bombay, Powai, Mumbai and SAIF, SPPU, Pune for providing characterization facilities.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Gg-g-An copolymer was synthesized by γ –irradiation method.
• Grafting of An onto Gg and modification to amidoximation Gg-g-AO were evidenced by FTIR, TGA and FESEM studies.
• Chelating Gg-g-AO has potential competence to recover uranyl ions from aqueous solution.
Rights and permissions
About this article
Cite this article
Shelar-Lohar, G., Joshi, S. Synthesis and characterization of gum ghatti grafted chelating copolymer for an effective removal of uranyl ions. J Polym Res 26, 179 (2019). https://doi.org/10.1007/s10965-019-1781-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-019-1781-x