Abstract
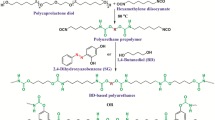
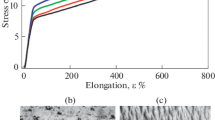
A series of the polyurethane materials with different ratios of hydroquinone ether derivatives and bisphenol A glycerolate diacrylate were synthesized using a two-step polymerization procedure. The structures of the obtained polyurethanes were characterized by Fourier transform infrared spectroscopy (FTIR). The influence of the hard segments structure on the thermo-mechanical properties were studied by TGA and stress-strain testing. It was found that with the increase in bisphenol A glycerolate diacrylate content, the new polyurethane materials had improved thermo-mechanical properties, due to the fact that the material develops a crosslinked structure. The durability of the polyurethane films when exposed to ultraviolet radiation was tracked by mechanical behavior and changes in chemical structure. Bisphenol A glycerolate diacrylate-based polyurethane elastomers exhibit improved behavior under UV radiation exposure compared with conventional polyurethanes that are chain-extended with butanediol.






Similar content being viewed by others
References
Chang Z, Zhang M, Hudson AG, Orler EB, Moore RB, Wilkes GL, Turner SR (2013) Synthesis and properties of segmented polyurethanes with triptycene units in the hard segment. Polymer 54:6910–6917
Oprea S (2010) Dependence of fungal biodegradation of PEG/castor oil-based polyurethane elastomers on the hard-segment structure. Polym Degrad Stabil 95:2396–2404
Oprea S (2011) Effect of the long chain extender on the properties of linear and castor oil cross-linked PEG-based polyurethane elastomers. J Mater Sci 46:2251–2258
Oprea S, Doroftei F (2011) Biodegradation of polyurethane acrylate with acrylated epoxidized soybean oil blend elastomers by Chaetomium globosum. Int Biodeterior Biodegrad 65:533–538
Oprea S, Gradinariu P, Joga A, Oprea V (2013) Synthesis, structure and fungal rezistance of sulfadiazine-based polyurethane ureas. Polym Degrad Stabil 98:1481–1488
Oprea S, Potolinca VO, Oprea V (2016) Synthesis and properties of new crosslinked polyurethane elastomers based on isosorbide. Eur Polym J 83:161–172
Oprea S, Potolinca VO, Gradinariu P, Joga A, Oprea V (2016) Synthesis, properties, and fungal degradation of castor-oil-based polyurethane composites with different cellulose contents. Cellulose 23(4):2515–2526
Kiran S, Joseph R (2014) Synthesis and characterization of a noncytotoxic, X-ray opaque polyurethane containing iodinated hydroquinone bis(2-hydroxyethyl) ether as chain extender for biomedical applications. J Biomed Mater Res Part A 102A:3207–3215
Oprea S (2013) Properties of crosslinked polyurethanes obtained by acrylic side-group polymerization and of their blends with various plant oils. J Appl Polym Sci:129–3640, 3649
Lin YH, Liao KH, Chou NK, Wang SS, Chu SH, Hsieh KH (2008) UV curable low surface energy fluorinated poly(urethaneacrylate)s for biomedical applications. Eur Polym J 44(9):2927–2937
He Y, Zhou M, Wu B, Jiang Z, Nie J (2010) Synthesis and properties of novel polyurethane acrylate containing 3(2hydroxyethyl) isocyanurate segment. Prog Org Coat 67(3):264–268
Liu X, Wang T, Li J, Cheng J, Zhang J (2015) Synthesis and properties of segmented polyurethanes with hydroquinone ether derivatives as chain extender. J Polym Res 22:149
Huang C-C, Chen Y-F, Suen S-Y, Lin C-H, Dai SA (2015) Synthesis and evaluation of alkoxylated-ether diols of hydroquinone with different chain-lengths as extenders in segmented polyurethanes. J Polym Res 22:171
Wang H, Wang Y, Liu D, Sun Z, Wang H (2014) Effects of additives on weather-resistance properties of polyurethane films exposed to ultraviolet radiation and ozone atmosphere. J Nanomater Article ID 487343
Hang TTX, Dung NT, Truc TA, Duong NT, Truoc BV, Vu PG, Hoang T, Thanh DTM, Olivier M-G (2015) Effect of silane modified nano ZnO on UV degradationof polyurethane coatings. Prog Org Coat 79:68–74
Rashvand M, Ranjbar Z (2014) Degradation and stabilization of an aromatic Polyurethane coating during an artificial aging test via FTIR spectroscopy. Mater Corros 65:76–81
Rekondo A, Fernandez-Berridi MJ, Irusta L (2007) Photooxidation and stabilization of silanised poly(ether-urethane) hybrid systems. Polym Degrad Stabil 92:2173–2180
Gao W, Wu Z, Wu Z, Iwashita K, Inagaki H (2012) Study on UV degradation resistance of a sand-fixing material by W-OH. J Soc Mater Sci, Jp 61(8):730–735
Searle ND (2003) In: Andrady AL (ed) Environmental effects on polymeric materials in Plastics and the Environment. John Wiley & Sons, Inc, Hoboken
França de Sa S, Ferreira JL, Pombo Cardoso I, Macedo R, Ramos AM (2017) Shedding new light on polyurethane degradation: Assessing foams condition in design objects. Polym Degrad Stab 144:354–365
Merlatti C, Perrin FX, Aragon E, Margaillan A (2008) Natural and artificial weathering characteristics of stabilized acrylic-urethane paints. Polym Degrad Stabil 93:896–903
Zha L, Wu M, Yang J (1999) Hydrogen bonding and morphological structure of segmented polyurethanes based on hydroquinone–bis(β-hydroxyethyl)ether as a chain extender. J Appl Polym Sci 73:2895–2902
Zhang Y, Shen Z, Yang D, Feng C, Hu J, Lu G, Huang X (2010) Convenient synthesis of PtBA-g-PMA well-defined graft copolymer with tunable grafting density. Macromolecules 43(1):117–125
Gao J, Ma Y, Liu H (2011) Uv-curable coating of epoxy-acrylate/ polyurethane acrylate nanocomposites modified with V-POSS. Adv Mater Res 217-218:559–563
Wang Y, Sun Z, Tian J, Wang H, Wang H, JI Y (2016) Influence of environment on ageing behaviour of the polyurethane film. Mater Sci 22: 290-294
Fang ZH, Shang JJ, Huang YX, Wang J, Li DQ, Liu ZY (2010) Preparation and characterization of the heat-resistant UV curable waterborne polyurethane coating modified by bisphenol A. Express Polym Lett 4(11):704–711
Bernard C, Nguyen T, Pellegrin B, Holbrook RD, Zhao M, Chin J (2011) Fate of graphene in polymer nanocomposite exposed to UV radiation. J Phys Conf Ser 304:012063
Davies P, Evrard G (2007) Accelerated ageing of polyurethanes for marine applications. Polym Degrad Stabil 92(8):1455–1464
McCrum NG, Buckley CP, Bucknall CB (1997) Principles of Polymer Engineering, second ed., OUP Inc., NY
Peinado C, Allen NS, Salvador EF, Corrales T, Catalina F (2002) Chemiluminescence and fluorescence for monitoring the photooxidation of an UV-cured aliphatic polyurethane-acrylate based adhesive. Polym Degrad Stab 77:523–529
Hu X, Zhang X, Liu J (2015) Synthesis and optical performances of a waterborne polyurethane-based polymeric dye. Int J Polym Sci Article ID 425083
Wang Y, Wang H, Li X, Liu D, Jiang Y, Sun Z (2013) O3/UV Synergistic aging of polyester polyurethane film modified by composite UV absorber. J Nanomater Article ID 169405, 7 pages
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Oprea, S., Potolinca, V.O. & Oprea, V. Influence of the hydroquinone ether moieties and Bisphenol A glycerolate diacrylate on the UV stability behavior of new polyurethane materials. J Polym Res 25, 79 (2018). https://doi.org/10.1007/s10965-018-1465-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-018-1465-y