Abstract
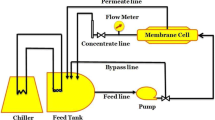
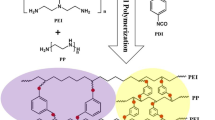
The research in this work exploits different diamines to crosslink chemically a polyamide thin layer to a PES/PI blend substrate through a new procedure. The main goal was to prepare innovative new polyamide (PA) thin film membranes without using an aqueous phase (unlike conventional methods to fabricate the PA thin layers) and to try chemically binding the formed thin layer to the substrate. Different diamines, namely EDA (ethylenediamine), PIP (piperazine), and PPD (para-phenylenediamine) were used as monomers and crosslinkers to open the imide rings and to provide the desired sites for better attaching of the generated thin layers to the support. Computational methods were used to assess the reaction mechanism and strength of the formed bindings. Chemical and physical properties of the modified and unmodified membranes were measured by SEM, AFM, FTIR-ATR, TGA, and contact angle test. The IR spectra and the measured performance indicated that the thin layer was successfully formed. The rejection capability of the membranes against Na2SO4 increased from near 2% to about 85% in the TMC-EDA modified membrane. Antifouling properties of the obtained membranes were measured by BSA solution and E. coli bacteria, showing a flux recovery ratio near 97% and favorable antibacterial properties for the TMC-EDA modified membrane. The calculation results also proved that the EDA was the best crosslinker and monomer for this purpose, and accordingly, the TMC-EDA modified membrane showed the desired binding strength.











Similar content being viewed by others
References
Eriksson P (1988) Water and salt transport through two types of polyamide composite membranes. J Membr Sci 36:297–313
Raman LP, Cheryna M, Rajagopalan N (1994) Consider nanofiltration for membrane separations. Chem Eng Prog 90(3):68–74
Cadotte J, Forester R, Kim M, Petersen R, Stocker T (1988) Nanofiltration membranes broaden the use of membrane separation technology. Desalination 70(1-3):77–88
Mansourpanah Y, Kakanejadifard A, Dehrizi FG, Tabatabaei M, Afarani HS (2015) Increasing and enhancing the performance and antifouling characteristics of PES membranes using acrylic acid and microwave-modified chitosan. Korean J Chem Eng 32(1):149–158
Lianchao L, Baoguo W, Huimin T, Tianlu C, Jiping X (2006) A novel nanofiltration membrane prepared with PAMAM and TMC by in situ interfacial polymerization on PEK-C ultrafiltration membrane. J Membr Sci 269(1):84–93
Verissimo S, Peinemann KV, Bordado J (2006) Influence of the diamine structure on the nanofiltration performance, surface morphology and surface charge of the composite polyamide membranes. J Membr Sci 279(1):266–275
Morgan PW (1965) Condensation polymers: by interfacial and solution methods (Vol. 10). Interscience Publishers, New York, pp 19–64
Bhattacharya A, Ray P, Brahmbhatt H, Vyas KN, Joshi SV, Devmurari CV, Trivedi JJ (2006) Pesticides removal performance by low‐pressure reverse osmosis membranes. J Appl Polym Sci 102(4):3575–3579
Jeong BH, Hoek EM, Yan Y, Subramani A, Huang X, Hurwitz G, Jawor A (2007) Interfacial polymerization of thin film nanocomposites: a new concept for reverse osmosis membranes. J Membr Sci 294(1):1–7
Freger V (2003) Nanoscale heterogeneity of polyamide membranes formed by interfacial polymerization. Langmuir 19(11):4791–4797
Song Y, Sun P, Henry LL, Sun B (2005) Mechanisms of structure and performance controlled thin film composite membrane formation via interfacial polymerization process. J Membr Sci 251(1):67–79
Mansourpanah Y, Jafari Z (2015) Efficacy of different generations and concentrations of PAMAM–NH 2 on the performance and structure of TFC membranes. React Funct Polym 93:178–189
Chen SH, Chang DJ, Liou RM, Hsu CS, Lin SS (2002) Preparation and separation properties of polyamide nanofiltration membrane. J Appl Polym Sci 83:1112–1118
Zhou Y, Yu S, Liu M, Gao C (2005) Preparation and characterization of polyamide-urethane thin-film composite membranes. Desalination 180(1):189–196
Liu LF, Yu SC, Wu LG, Gao CJ (2008) Study on a novel antifouling polyamide–urea reverse osmosis composite membrane (ICIC–MPD): III. Analysis of membrane electrical properties. J Membr Sci 310(1):119–128
Singh PS, Joshi SV, Trivedi JJ, Devmurari CV, Rao AP, Ghosh PK (2006) Probing the structural variations of thin film composite RO membranes obtained by coating polyamide over polysulfone membranes of different pore dimensions. J Membr Sci 278(1):19–25
Ghosh AK, Hoek EM (2009) Impacts of support membrane structure and chemistry on polyamide–polysulfone interfacial composite membranes. J Membr Sci 336(1):140–148
Mansourpanah Y, Gheshlaghi A, Rekabdar F (2012) Structural analysis of PES nanoporous membranes under different conditions of preparation. Desalin Water Treat 50(1-3):302–309
Rahimpour A, Madaeni SS (2007) Polyethersulfone (PES)/cellulose acetate phthalate (CAP) blend ultrafiltration membranes: preparation, morphology, performance and antifouling properties. J Membr Sci 305(1):299–312
Mansourpanah Y, Madaeni SS, Rahimpour A, Kheirollahi Z, Adeli M (2010) Changing the performance and morphology of polyethersulfone/polyimide blend nanofiltration membranes using trimethylamine. Desalination 256(1):101–107
Vanherck K, Koeckelberghs G, Vankelecom IF (2013) Crosslinking polyimides for membrane applications: a review. Prog Polym Sci 38(6):874–896
Kapantaidakis GC, Koops GH (2002) High flux polyethersulfone–polyimide blend hollow fiber membranes for gas separation. J Membr Sci 204(1):153–171
Powell CE, Duthie XJ, Kentish SE, Qiao GG, Stevens GW (2007) Reversible diamine cross-linking of polyimide membranes. J Membr Sci 291(1):199–209
Liu Y, Wang R, Chung TS (2001) Chemical cross-linking modification of polyimide membranes for gas separation. J Membr Sci 189(2):231–239
Qiao X, Chung TS (2006) Diamine modification of P84 polyimide membranes for pervaporation dehydration of isopropanol. AIChE J 52(10):3462–3472
Ulbricht M (2006) Advanced functional polymer membranes. Polymer 47(7):2217–2262
Shao L, Chung TS, Goh SH, Pramoda KP (2005) Polyimide modification by a linear aliphatic diamine to enhance transport performance and plasticization resistance. J Membr Sci 256(1):46–56
Tin PS, Chung TS, Liu Y, Wang R, Liu SL, Pramoda KP (2003) Effects of cross-linking modification on gas separation performance of Matrimid membranes. J Membr Sci 225(1):77–90
Cao C, Chung TS, Liu Y, Wang R, Pramoda KP (2003) Chemical cross-linking modification of 6FDA-2, 6-DAT hollow fiber membranes for natural gas separation. J Membr Sci 216(1):257–268
Liu Y, Chung TS, Wang R, Li DF, Chng ML (2003) Chemical cross-linking modification of polyimide/poly (ether sulfone) dual-layer hollow-fiber membranes for gas separation. Ind Eng Chem Res 42(6):1190–1195
Shao L, Chung TS, Goh SH, Pramoda KP (2004) Transport properties of cross-linked polyimide membranes induced by different generations of diaminobutane (DAB) dendrimers. J Membr Sci 238(1):153–163
Zhao HY, Cao YM, Ding XL, Zhou MQ, Liu JH, Yuan Q (2008) Poly (ethylene oxide) induced cross-linking modification of Matrimid membranes for selective separation of CO2. J Membr Sci 320(1):179–184
Zhao HY, Cao YM, Ding XL, Zhou MQ, Yuan Q (2008) Effects of cross-linkers with different molecular weights in cross-linked Matrimid 5218 and test temperature on gas transport properties. J Membr Sci 323(1):176–184
Solomon MFJ, Bhole Y, Livingston AG (2012) High flux membranes for organic solvent nanofiltration (OSN)—Interfacial polymerization with solvent activation. J Membr Sci 423:371–382
Toh YS, Lim FW, Livingston AG (2007) Polymeric membranes for nanofiltration in polar aprotic solvents. J Membr Sci 301(1):3–10
Wang YQ, Su YL, Ma XL, Sun Q, Jiang ZY (2006) Pluronic polymers and polyethersulfone blend membranes with improved fouling-resistant ability and ultrafiltration performance. J Membr Sci 283(1):440–447
Alkorta I, Blanco F, Elguero J (2010) Dihydrogen bond cooperativity in aza-borane derivatives. J Phys Chem A 114(32):8457–8462
Atkins PW, Friedman RS (2011) Molecular quantum mechanics. Oxford university press
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA et al (2009) Gaussian. Gaussian. Inc, Wallingford
Bader RFW (1990) Atoms in molecules: a quantum theory. Oxford University Press, Oxford
Karas BR, Foust DF, Dumas WV, Lamby EJ (1992) Aqueous pretreatments of polyetherimide to facilitate the bonding of electrolessly deposited copper. J Adhes Sci Technol 6(7):815–828
Nadler R, Srebnik S (2008) Molecular simulation of polyamide synthesis by interfacial polymerization. J Membr Sci 315(1):100–105
Freger V (2005) Kinetics of film formation by interfacial polycondensation. Langmuir 21(5):1884–1894
Perrin DD (1972) Dissociation constants of organic bases in aqueous solution: supplement, Butterworths
Dennington R, Keith T, Millam J (2009) GaussView version 5. Semichem Inc, U.S
Kim IC, Lee KH (2002) Preparation of interfacially synthesized and silicone-coated composite polyamide nanofiltration membranes with high performance. Ind Eng Chem Res 41:5523–5528
Shao L, Liu L, Cheng SX, Huang YD, Ma J (2008) Comparison of diamino cross-linking in different polyimide solutions and membranes by precipitation observation and gas transport. J Membr Sci 312:174–185
Dietz P, Hansma PK, Inacker O, Ehmann HD, Herrmann KH (1992) Surface pore structures of micro- and ultrafiltration membranes imaged with the atomic force microscope. J Membr Sci 65:101–111
Mansourpanah Y, Madaeni SS, Adeli M, Rahimpour A, Farhadian A (2009) Surface modification and preparation of nanofiltration membrane from polyethersulfone/polyimide blend-use of a new material (Polyethyleneglycol-Triazine). J Appl Polym Sci 112:2888–2895
Zabaradsti A, Kakanejadifard A, Ghasemian G (2012) Theoretical study of molecular interactions of phosphorus ylide with hypohalous acids HOF, HOCl and HOBr. Comput Theor Chem 989:1–6
Basser MA, Mote NA (2001) Synthesis and antimicrobial activity of some Schiff bases from benzothiazoles. Asian J Chem 13:496–500
Acknowledgements
We would like to thank Dr. H. Shamloei (Computational Research Laboratory, Lorestan University) for his guidance in computational assessments throughout the work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Highlights
• Preparation of a polymeric thin film membrane in a new procedure
• To try chemically attaching the formed thin layer to the PES/PI support
• Using different diamines as monomer and cross-linker to open the imide rings in PI
• Increasing the rejection and antifouling as well as antibacterial properties of the membranes
• Using computational methods to investigate the strength of the formed bindings in thin layer
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 261 kb)
Rights and permissions
About this article
Cite this article
Mansourpanah, Y., Ostadchinigar, A. Preparation of chemically attached polyamide thin film membrane using different diamines: separation and computational investigation. J Polym Res 24, 26 (2017). https://doi.org/10.1007/s10965-017-1186-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-017-1186-7