Abstract
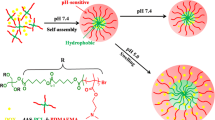
Novel double hydrophilic poly(monomethylitaconate)-co-poly(N,N-dimethylaminoethyl methacrylate) (PMMI-co-PDMAEMA) synthesized via free radical polymerization of corresponding monomers with defined molar ratios in the presence of K2S2O8 as an initiator in an aqueous solution. The resulting copolymer was subsequently converted to a cholesterol conjugate (PMMICholC6-co-PDMAEMA) by esterification reaction with 6-cholesteryl-1-hexanol (CholC6). Two copolymers self-assembled into micelles by simply adjusting the solution pH at room temperature. The non-conjugated polymer had a sharp transition at pH 5. TEM and DLS studies showed that both micelles were spherical in shape with a mean diameter around 85 and 26 nm, respectively. Piroxicam (PX) as a hydrophobic model drug was encapsulated into micelles. The results indicated that PMMICholC6-co-PDMAEMA micelles were able to load more amounts of drug than PMMI-co-PDMAEMA micelles which could be attributed to the strong hydrophobic interactions of cholesterol molecules in the core. In vitro release studies demonstrated that PX release from PMMI-co-PDMAEMA micelles was significantly fast at physiological pH compared with mildly acidic pH 4.5. However, at pH 4.5, PMMICholC6-co-PDMAEMA micelles, with core-shell-corona structure, released loaded drug molecules faster than pH 7.4 which contained a relatively steady drug release profile. In summary, cholesterol-modified micelles could be introduced as stable and effective pH responsive nanocarriers to make a promising system for enhancing the efficacy of hydrophobic drugs in cancer cells for improved cancer therapy.










Similar content being viewed by others
References
Cölfen H (2001) Double-hydrophilic block copolymers: synthesis and application as novel surfactants and crystal growth modifiers. Macromol Rapid Commun 22:219–252
Bütün V, Billingham NC, Armes SP (1998) Unusual aggregation behavior of a novel tertiary amine methacrylate-based diblock copolymer: formation of micelles and reverse micelles in aqueous solution. J Am Chem Soc 120:11818–11819
Zhao C, He P, Xiao C, Gao X, Zhuang X, Chen X (2011) Synthesis of temperature and pH-responsive crosslinked micelles from polypeptide-based graft copolymer. J Colloid Interface Sci 359:436–442
Dua J, O’Reilly RK (2009) Advances and challenges in smart and functional polymer vesicles. Soft Matter 5:3544–3561
Rodríguez-Hernández J, Lecommandoux S (2005) Reversible inside-out micellization of pH-responsive and water-soluble vesicles based on polypeptide diblock copolymers. J Am Chem Soc 127:2026–2027
Liu S, Armes SP (2002) Polymeric surfactants for the new millennium: a ph-responsive, zwitterionic, schizophrenic diblock copolymer. Angew Chem Int Ed 41:1413–1416
Bories-Azeau X, Armes SP, van den Haak HJW (2004) Synthesis and aqueous solution properties of novel sugar methacrylate-based homopolymers and block copolymers. Macromolecules 37:2348–2352
Li G, Shi L, Ma R, An Y, Huang N (2006) Formation of complex micelles with double-responsive channels from self-assembly of two diblock copolymers. Angew Chem Int Ed 45:4959–4962
Willet N, Gohy JF, Auvray L, Varshney S, Jérôme R, Leyh B (2008) Core-shell-corona micelles by PS-b-P2VP-b-PEO copolymers: focus on the water-induced micellization process. Langmuir 24:3009–3015
Li G, Guo L, Ma S, Liu J (2009) Complex micelles formed from two diblock copolymers for applications in controlled drug release. J Polym Sci A Polym Chem 47:1804–1810
Guo XD, Zhang LJ, Chen Y, Qian Y (2010) Core/shell pH-sensitive micelles self-assembled from cholesterol conjugated oligopeptides for anticancer drug delivery. AICHE J 56:1922–1931
Wetering PVD, Cherng JY, Talsma H, Crommelin DJA, Hennink WE (1998) 2-(dimethylamino)ethyl methacrylate based (co)polymers as gene transfer agents. J Control Release 53:145–153
Mao J, Ji X, Bo S (2011) Synthesis and pH/temperature-responsive behavior of PLLA-b PDMAEMA block polyelectrolytes prepared via ROP and ATRP. Macromol Chem Phys 212:744–752
Bütün V, Bennett CE, Vamvakaki M, Lowe AB, Billingham NC, Armes SP (1997) Selective betainisation of tertiary amine methacrylate block copolymers. J Mater Chem 7:1693–1695
Maoa BW, Gana LH, Gana YY, Tamb KC, Tanc OK (2005) Controlled one-pot synthesis of pH-sensitive self-assembled diblock copolymers and their aggregation behavior. Polymer 46:10045–10055
Jiang X, Ge Z, Xu J, Liu H, Liu S (2007) Fabrication of multiresponsive shell cross-linked micelles possessing pH-controllable core swellability and thermo-tunable corona permeability. Biomacromolecules 10:3184–3192
Du J, Tng Y, Lewis AL, Armes SP (2005) pH-Sensitive vesicles based on a biocompatible zwitterionic diblock copolymer. J Am Chem Soc 127:17982–17983
Fujii S, Cai Y, Weaver JWM, Armes SP (2005) Syntheses of shell cross-linked micelles using acidic ABC triblock copolymers and their application as pH-responsive particulate emulsifiers. J Am Chem Soc 127:7304–7305
Kurata K, Dobashi A (2004) Novel temperature and pH-responsive linear polymers and crosslinked hydrogels comprised of acidic L-α-amino acid derivatives. J Macromol Sci A Pure Appl Chem 41:143–164
Li GY, Shi LQ, An YL (2006) Double-responsive core-shell-corona micelles from self-assembly of diblock copolymer of poly (t-butyl acrylate-co-acrylic acid)-b-poly (N-isopropylacryla-mide). Polymer 47:4581–4587
Bagheri M, Pourmoazzen Z, Entezami AA (2013) Synthesis, characterization and liquid crystalline behavior of poly(monomethyl itaconate)s with new pendant cholesterol moieties. Iran Polym J 22:303–311
Yoo MK, Sung YK, Cho CS, Lee YM (1997) Effect of polymer complex formation on the cloud-point of poly(N-isopropyl acrylamide) (PNIPAAm) in the poly(NIPAAm-co-acrylic acid): polyelectrolyte complex between poly(acrylic acid) and poly(allylamine). Polymer 38:2759–2765
Yinsonga W, Lingrong L, Jian W, Zhang W (2007) Preparation and characterization of self-aggregated nanoparticles of cholesterol-modiWed O-carboxymethyl chitosan conjugates. Carbohydr Polym 69:597–606
Yang L, Zhang B, Wen L, Liang O, Zhang LM (2007) Amphiphilic cholesteryl grafted sodium alginate derivative: synthesis and self-assembly in aqueous solution. Carbohydr Polym 68:218–225
Zhou Y, Briand VA, Sharma N, Ahn SK, Kasi RM (2009) Polymers comprising cholesterol: synthesis, self-Assembly, and applications. Materials 2:636–660
Dimitrov I, Trzebicka B, Müller AHE, Dworak A, Tsvetanov CB (2007) Thermosensitive water-soluble copolymers with doubly responsive reversibly interacting entities. Prog Polym Sci 32:1275–1343
McCormick CL, Kirkland SE, York AW (2006) Synthetic routes to stimuli-responsive micelles, vesicles, and surfaces via controlled/living radical polymerization. Polym Rev 46:421–443
Cui W, Lu X, Cui K, Niu L, Wei Y, Lu O (2012) Dual-responsive controlled drug delivery based on ionically assembled nanoparticles. Langmuir 28:9413–9420
Xin B, Yokoyama Y, Shigeto T, Mizunuma H (2007) Anti-tumor effect of non-steroidal anti-inflammatory drugs on human ovarian cancers. Pathol Oncol Res 13:365–369
Acknowledgments
The authors would like to thank Research Vice Chancellor of Azarbaijan Shahid Madani University for financial support of this research. The authors’ warm thanks are also extended to Dr. H. Razmi and Mr. Rahim Mohammad-Rezaei for their useful spectroscopic assistances and Mr. B. Ramazani for him English assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bagheri, M., Pourmoazzen, Z. & Entezami, A.A. pH-responsive nanosized-micelles based on poly(monomethylitaconate)-co-poly(dimethylaminoethyl methacrylate) and cholesterol side chains effect on pH change-induced release of piroxicam. J Polym Res 20, 231 (2013). https://doi.org/10.1007/s10965-013-0231-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-013-0231-4