Abstract
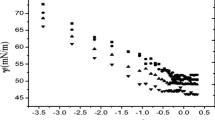
The micellar and associative properties of four diblock copolymers, Me2N(CH2)2OE79B34 (denoted DE80B34), I−Me3N+(CH2)2OE79B34 (denoted TE80B34), I−Me3N+(CH2)2OE48B22 (denoted TE49B22), and HO(CH2)2OE62B22 (denoted E62B22), in aqueous solution and at various concentrations and temperatures, were investigated by surface tensiometry and dynamic and static laser light scattering. Surface tension measurements enabled the critical micelle concentration (CMC) to be determined at different temperatures, and thus the enthalpy of micellization (ΔH o mic), the free energy of micellization (ΔG o mic), and the entropy of micellization (ΔS o mic) to be ascertained. Dynamic and static light-scattering measurements allowed the micellar parameters to be calculated and the extent of hydration of the copolymer micelle to be obtained qualitatively. The experimental results provided by these techniques are discussed in the terms of the variation in the hydrophilic to hydrophobic (E/B) ratio and end-group modification. Micellar parameters such as the weight-average molar mass (M w), the association number (N w), the thermodynamic radius (r t), and the hydrodynamic radius (r h) obtained from light-scattering data show that the micelles formed by the conventional E m B n and dimethylamino-tipped (DE m B n ) copolymers are harder than those of trimethylammonium-tipped (TE m B n ) copolymers. This difference in micellar properties is considered to be due to differences in polarity and charge effect at the hydrophilic ends of the tip-modified copolymers. The high value of r h for DE m B n and TE m B n copolymers as compared to E62B22 is an indication of micellar aggregation.






Similar content being viewed by others
References
Hadjichristidis N, Pispas S, Floudas GA (2003) Block copolymers. Synthetic strategies, physical properties and applications. Wiley, New York
Rao J, Zhang J, Xu J, Liu S (2008) J Colloid Interface Sci 328:196–202
Hamley W (1998) The physics of block copolymers. Oxford University Press, Oxford
Jones MC, Gao H, Leroux C (2008) J Control Release 132:208–215
Booth C, Attwood D (2000) Macromol Rapid Commun 21:501–527
Loh W (2002) In: Hubbard AT (ed) Encyclopedia of surface and colloid science. Marcel Dekker, New York, pp 802–813
Cambón A, Rey-Rico A, Barbosa S, Soltero JFA, Yeates SG, Brea J, Loza MI, Alvarez-Lorenzo C, Concheiro A, Taboada P, Mosquera V (2013) J Control Release 167:68–75
Yu K, Esenberg A (1998) Macromolecules 31:3509–3518
Liu G (2000) Chin J Polym Sci 18:255–262
Spatz JP, Herzog T, Mobmer S, Ziemann P, Moller M (1999) Adv Mater 11:149–153
Jenekhe SA, Chen XL (1999) Science 283:372–375
Iijma M, Nagasaki Y, Okada T, Kato M, Kataoka K (1999) Macromolecules 2:1140–1146
Otsuka U, Nagasaki Y, Kataoka K, Okano T, Sakurai Y (1998) Polym Prepr 9:128–129
Yuan J, Xu Z, Cheng S, Feng L (2002) Eur Polym J 38:1537–1546
Booth C, Yu GE, Nace VM (2000) In: Alexandridis P, Lindman B (eds) Amphiphilic block copolymers: self-assembly and applications. Elsevier, Amsterdam, pp 57–86
Booth C, Attwood D, Price C (2006) Phys Chem Chem Phys 8:3612–3622
Alexandridis P (1997) Curr Opin Colloid Interface Sci 2:478–489
Castro E, Tabooda P, Mosquera V (2005) J Phys Chem B 109:5592–5599
Li X, Wettig SD, Verrall RE (2005) J Colloid Interface Sci 282:466–477
Mata J, Joshi T, Varade D, Ghosh G, Bahadur P (2004) Colloids Surf A 247:1–7
Desai H, Varade D, Aswal VK, Goyal PS, Bauhaus P (2006) Eur Polym J 42:593–601
Jain NJ, Aswal VK, Goyal PS, Bahadur P (2000) Colloids Surf A 173:85–94
Castro E, Tabooda P, Mosquera V (2006) J Phys Chem B 110:13113–13123
Ganguly R, Aswal VK, Hassan PA, Gopalakrishnan IK, Yakhmi JV (2005) J Phys Chem B 109:5653–5658
Kelarakis A, Mai SM, Havredaki V, Nace VM, Booth C (2001) Phys Chem Chem Phys 3:4037–4043
Maskos M (2006) Polymer 47:1172–1178
Tattershall CE, Jerome NP, Budd PM (2001) J Mater Chem 11:2979–2984
Tattershall CE, Aslam SJ, Budd PM (2002) J Mater Chem 12:2286–2291
Siddiq M, Harrison W, Tattershall CE, Budd PM (2003) Phys Chem Chem Phys 5:3968–3972
Rosen MJ (1978) Surfactants and interfacial phenomena. Wiley, New York, pp 83–89
Khan A, Siddiq M (2010) J Appl Polym Sci 118:3324–3332
Hall DG (1987) In: Schick MJ (ed) Nonionic surfactants: physical chemistry. Marcel Dekker, New York, pp 247–248
Sultana SB, Bhat SGT, Rakshit AK (1997) Langmuir 13:4562–4568
Nostro PL, Gabrielli G (1993) Langmuir 9:3132–3137
Soni SS, Sastry NV, Patra AK, Joshi JV, Goyal PS (2002) J Phys Chem B 106:13069–13077
Soni SS, Sastry NV, Aswal VK, Goyal PS (2002) J Phys Chem B 106:2606–2617
Rosen MJ, Cohen AW, Dahanayeke M, Hua X-Y (1982) J Phys Chem 86:541–545
Hiemenz PC, Rajagopalan R (1997) Principles of colloids and surface chemistry, 3rd edn. Marcel Dekker, New York
William RJ, Phillips JN, Mysels KJ (1955) Trans Faraday Soc 51:561–569
Provencher SW (1979) Makromol Chem 180:201–209
Chaibundit C, Ricardo NMPS, Crothers M, Booth C (2002) Langmuir 18:4277–4283
Barbosa S, Cheema MA, Taboada P, Mosquera V (2007) J Phys Chem B 111:10920–10928
Wu C, Xia KQ (1994) Rev Sci Instrum 65:587–590
Vrij A (1978) J Chem Phys 69:1742–1747
Mai SM, Booth C, Nace VM (1997) Eur Polym J 33:991–996
Khan A, Farooqi ZH, Siddiq M (2012) J Appl Polym Sci 124:951–957
Siddiq M, Liu G, Zhang G, Khan A, Budd PM (2010) Polym Bull 65:521–531
Derici L, Ledger S, Mai SM, Booth C, Hamley IW, Pedersen JS (1999) Phys Chem Chem Phys 1:2773–2785
Mingavinish W, Mai SM, Heatle F, Booth C (1999) J Phys Chem B 103:11269–11274
Hamley IW, Daniel C, Mingvanish W, Mai SM, Booth C, Messe L, Ryan AJ (2000) Langmuir 16:2508–2514
Budd PM (1989) In: Allen G, Bevington JC, Booth C, Price C (eds) Comprehensive polymer science. Pergamon, Oxford
Budd PM (2002) In: Tripathy SK, Kumar J, Nalwa HS (eds) Handbook of polyelectrolytes and their applications. American Scientific, Stevenson Ranch
James AM, Lord MP (1992) Macmillan’s chemical and physical data. Macmillan, London
Diaz-Fernandez Y, Foti F, Mangano C, Pallavicini P, Patroni S, Perez-Gramatges A, Rodriguez-Calvo S (2006) Chem Eur J 12:921–930
Acknowledgments
We are very grateful to Dr. Carin Tattershall (University of Manchester) for the synthesis of the dimethylamino- and trimethylammonium-tipped diblock copolymers. We are also thankful to Professor Peter M. Budd of the University of Manchester for helpful discussions. Dr. Abbas Khan is grateful to the Higher Education Commission (H.E.C.) Pakistan for financial support under the indigenous Ph.D. fellowship scheme. He also wishes to acknowledge the Third World Academy of Sciences for a split Ph.D. research fellowship to work in the Department of Chemical Physics, University of Science and Technology, Hefei, China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khan, A., Siddiq, M. Light scattering and surface tensiometric studies of tip-modified PEO-PBO diblock copolymers in water. J Polym Res 20, 160 (2013). https://doi.org/10.1007/s10965-013-0160-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-013-0160-2