Abstract
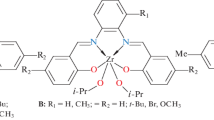
Method of polymerization quenching with radioactive carbon monoxide (14СО) has been used to determine the number of active centers (С P ) and propagation rate constants (k P ) for ethylene polymerization over the homogeneous catalysts 2,6-R2LVCl3+MAO (L: 2,6-(C6H3N=CMe)2C5H3N, R=Me, Et). For both catalysts the maximum number of active centers at 60 °C was found to be 0.42–0.50 mol molV −1 and the k P values—(16.4–20.0) × 103 L mol−1 s−1. It has been shown that noticeable decrease in the catalysts activity with polymerization time results from the decrease in the active centers number and simultaneous transformation of some part of the initial centers into a new ones with lower reactivity. Analysis of the С P and k P values, measured at different polymerization times, together with the values of the polyethylene molecular-weight distribution, allowed to calculate the content of the active centers of both types and the k P values of these centers. For the 2,6-Et2LVCl3+МАО catalyst, the effect of polymerization temperature on the С P and k P values has been studied. It has been found that with polymerization temperature growth from 25 to 60 °С, the С P value substantially increases. As a result, the value of the effective activation energy (E eff = 22.6 kcal mol−1) noticeably exceeded that of the activation energy of propagation reaction (E P = 14.2 kcal mol−1). It is proposed that the reason for the unusually high E P value is formation of “dormant” sites with vanadium-polymer bond at lower polymerization temperature.






Similar content being viewed by others
References
Britovsek GJ, Gibson VC, Kimberley BS, Maddox PJ, McTavish SJ, Solan GA, White AJ, Williams DJ (1998) Chem Commun 7:849–850
Small BL, Brookhart M, Bennet AM (1998) J Am Chem Soc 120:4049–4050
Britovsek GJP, Bruse M, Gibson VC, Kimberley BS, Maddox PJ, Mastroianni S, McTavish SJ, Redshaw C, Solan GA, Stromberg S, White AJ, Williams DJ (1999) J Am Chem Soc 121:8728–8740
Kumar KR, Sivaram S (2000) Macromol Chem Phys 210:1513–1520
Semikolenova NV, Zakharov VA, Talsi EP, Babushkin DE, Sobolev AP, Echevskaya LG, Khusniyarov MM (2002) J Mol Catal A Chem 182:283–294
Talsi EP, Babushkin DE, Semikolenova NV, Zudin VN, Panchenko VN, Zakharov VA (2001) Macromol Chem Phys 202:2046–2051
Bryliakov KP, Semikolenova NV, Zakharov VA, Talsi EP (2004) Organometallics 23:5375–5378
Britovsek GJP, Clentsmith GKB, Gibson VC, Goodgame DML, McTavish SJ, Pankhurst QA (2002) Catal Commun 3:207–211
Bart SC, Chlopek K, Bill E, Bouwkamp MW, Lobkovsky E, Neese F, Wieghardt K, Chirik PJ (2006) J Am Chem Soc 128:13901–13912
Castro PM, Lahtinen P, Axenov K, Viidanoja J, Kotiaho T, Leskela M, Repo T (2005) Organometallics 24:3664–3670
Humphries MJ, Tellmann KP, Gibson VC, White AJP, Williams DJ (2005) Organometallics 24:2039–2050
Kooistra TM, Knijnenburg Q, Smits JMM, Horton AD, Budzelaar PHM, Gal AW (2001) Angew Chem Int Ed 40:4719–4722
Soshnikov IE, Semikolenova NV, Bushmelev AN, Bryliakov KP, Lyakin OY, Redshaw C, Zakharov VA, Talsi EP (2009) Organometallics 28:6003–6013
Barabanov AA, Bukatov GD, Zakharov VA, Semikolenova NV, Echevskaja LG, Matsko MA (2005) Macromol Chem Phys 206:2292–2298
Barabanov AA, Bukatov GD, Zakharov VA, Semikolenova NV, Echevskaja LG, Matsko MA (2008) Macromol Chem Phys 209:2510–2515
Ittel SD, Johnson LK, Brookhart M (2000) Chem Rev 100:1169–1203
Gibson VC, Spitzmesser SK (2003) Chem Rev 103:283–315
Gibson VC, Redshaw C, Solan GA (2007) Chem Rev 107:1745–1776
Abu-Surrah A, Ibrahim KA, Abdalla MY, Issa AA (2011) J Polym Res 18:59–66
Damavandi S, Galland GB, Zohuri GH, Sandaroos R (2011) J Polym Res 18:1059–1065
Damavandi S, Zohuri GH, Sandaroos R, Ahmadjo S (2012) J Polym Res 19:9796
Jiang H, He F, Wang H (2009) J Polym Res 16:183–189
Cui L, Yu J, Lv Y, Li G, Zhou S (2012) J Polym Res 19:9881
Barabanov AA, Semikolenova NV, Matsko MA, Echevskaya LG, Zakharov VA (2010) Polymer 51:3354–3359
Reardon D, Conan F, Gambarotta S, Yap G, Wang QY (1999) J Am Chem Soc 121:9318–9325
Schmidt R, Welch MB, Knudsen RD, Gottfried S, Alt HG (2004) J Mol Catal A Chem 222:9–15
Schmidt R, Welch MB, Knudsen RD, Gottfried S, Alt HG (2004) J Mol Catal A Chem 222:17–25
Schmidt R, Das PK, Welch MB, Knudsen RD (2004) J Mol Catal A Chem 222:27–45
Colamarco E, Milione S, Cuomo C, Grassi A (2004) Macromol Rapid Commun 25:450–454
Lang JRV, Denner CE, Alt HG (2010) J Mol Catal A Chem 322:45–49
Semikolenova NV, Zakharov VA, Echevskaja LG, Matsko MA, Bryliakov KP, Talsi EP (2009) Catal Today 144:334–340
Wu J, Pan Q, Rempel GL (2005) J Appl Polym Sci 96:645–649
Natta G, Pasquon I (1959) Adv Catal 11:1–66
Barabanov AA, Bukatov GD, Zakharov VA (2008) J Polym Sci A Polym Chem 46:6621–6629
Zakharov VA, Chumaevskii NB, Bukatov GD, Ermakov YI (1976) Makromol Chem 177:763–775
Zakharov VA, Ermakov YI (1971) J Polym Sci A1 Polym Chem 9:3129–3146
Acknowledgments
This work was supported by the Russian Foundation of Basic Research, Grant 07-03-00311. The authors wish to thank Dr. L.G. Echevskaya for the study of PE MWD.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Barabanov, A.A., Semikolenova, N.V., Bukatov, G.D. et al. Ethylene polymerization over homogeneous Bis(imino)pyridine vanadium catalysts: data on the number and reactivity of active sites. J Polym Res 19, 9998 (2012). https://doi.org/10.1007/s10965-012-9998-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-012-9998-y