Abstract
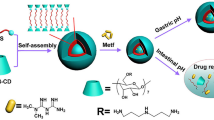
This study designed a series of polyanionic nanocarriers based on biodegradable and biocompatible poly (aspartic acid)s for oral administration. First, polysuccinimide (PSI) was synthesized from L-aspartic acid using acid-catalyzed bulk thermal polycondensation and acid-catalyzed thermal polycondensation in a mixture of mesitylene/sulfolane. PSI-C16 was then synthesized by aminolysis with nucleophile, hexadecylamine to react with PSI known as nucleophilic addition. Finally, a series of partially esterified poly (aspartic acid)s was produced by alkaline treatment to afford an amphiphilic polyanion, poly (sodium aspartate-g-hexadecyl aspartate) (Na-PASP-g-C16-PASP). 1HNMR, FTIR, DSC and GPC were utilized to demonstrate and characterize the polymers. The synthesized polyanion could be self-assembled into the nano-scaled micelles and be independent of pH in phosphoric buffer solutions. The hydrodynamic diameter and zeta potential were measured using the dynamic light scattering (DLS) method, and the critical micelle concentration (CMC) was determined using the fluorescence spectrophotometer. The micellar morphologies were examined using transmission electron microscopy (TEM), and atomic force microscopy (AFM) to present the nano-dimensional sphere. The stability of size transition at different pH levels, from strong acid to alkaline, proved that the micelles could stably transport from the stomach to intestinal lumen prior to arriving in the epithelium of the small intestine.















Similar content being viewed by others
References
Bromberg L (2008) J Control Rel 128:99
Francis MF, Cristea M, Winnik FM (2004) Pure Appl Chem 76:1321
Eiamtrakarn S, Itoh Y, Kishimoto J, Yoshikawa Y, Shibata N, Murakami M, Takada K (2002) Biomaterials 23:145
Koo OM, Rubinstein I, Onyuksel H (2005) Nanomed 1:193
Li H, Zhao X, Ma Y, Zhai G, Li L, Lou H (2009) J Control Rel 133:238
Kalaria DR, Sharma G, Beniwal V, Ravi KMN (2009) Pharm Res 26:492
Ling SS, Magosso E, Khan NA, Yuen KH, Barker SA (2006) Drug Dev Ind Pharm 32:335
Tang BC, Dawson M, Lai SK, Wang YY, Suk JS, Yang M, Zeitlin P, Boyle MP, Fu J, Hanes J (2009) Proc Nat Acad Sci 106:19268
Venkatesan N, Uchino K, Amagase K, Ito Y, Shibata N, Takada K (2006) J Control Rel 111:19
Ke W, Zhao Y, Huang R, Jiang C, Pei Y (2008) J Pharm Sci 97:2208
Suh J, Dawson M, Hanes J (2005) Adv Drug Deliv Rev 57:63
Dawson M, Wirtz D, Hanes J (2003) J Biol Chem 278:50393
Lai SK, Wang YY, Wirtz D, Hanes J (2009) Adv Drug Deliv Rev 61:158
Singh R, Lillard JW Jr (2009) J Exp Mol Pathol 86:215
Mauludin R, Muller RH, Keck CM (2009) Int J Pharm 370:202
Pandita D, Ahuja A, Velpandian T, Lather V, Dutta T, Khar RK (2009) Pharmazie 64:301
Jani P, Halbert GW, Langridge J, Florence AT (1990) J Pharm Pharmacol 42:821
Cai Z, Wang Y, Zhu LJ, Liu ZQ (2010) Curr Drug Metab 11:197
Woitiski CB, Carvalho RA, Ribeiro AJ, Neufeld RJ, Veiga F (2008) Bio Drugs 22:223
Florence AT (2005) Drug Discov Today 2:75
Desai MP, Labhasetwar V, Walter E, Levy RJ, Amidon GL (1997) Pharm Res 14:1568
Desai MP, Labhasetwar V, Amidon GL, Levy RJ (1996) Pharm Res 13:1838
Singh R, Singh S, Lillard JW Jr (2008) J Pharm Sci 97:2497
McDowell A, McLeod BJ, Rades T, Tucker IG (2009) N Z Vet J 57:370
Cui F, Qian F, Zhao Z, Yin L, Tang C, Yin C (2009) Biomacromolecules 10:1253
Cone RA (2009) Adv Drug Deliv Rev 61:75
Van KBJ, Dekker J, Buller HA, Einerhand AW (1995) Am J Physiol 269:G613
Corfield AP, Carroll D, Myerscough N, Probert CS (2001) Front Biosci 6:D1321
Mathiowitz E, Jacob JS, Jong YS, Carino GP, Chickering DE, Chaturvedi P et al (1997) Nature 386:410
Kriwet B, Walter E, Kissel T (1998) J Control Rel 56:149
Crater JS, Carrier RL (2010) Macromol Biosci 10:1473
Tachibana Y, Kurisawa M, Uyama H, Kobayashi S (2003) Chem Commun 1:106
Nakato T, Oda K, Yoshitake M, Tomida M (1999) J Macro Sci Pure Appl Chem 36:949
Watanabe E, Tomoshige N, Uyama H (2007) Macromol Symp 249:509
Neri P, Antoni G, Benvenuti F, Cocola F, Gazzei JG (1973) J Med Chem 16:893
Thombre SM, Sarwade BD (2005) J Macro Sci. Part A: Pure Appl Chem 42:1299
Yang J, Wang F, Fang L, Tan TW (2007) Environ Pollut 149:125
Zhang W, Huang J, Fan N, Yu J, Liu Y, Liu S et al (2010) Colloids Surface B: Biointerface 81:297
Obst M, Steinbuchel A (2004) Biomacromolecules 5:1166
Richard AG, Bhanu K (2002) Science 297:803
Nariyoshi K, Hidetaka H, Hidetoshi O (1995) J Ferment Bioeng 79:317
Wang J, Chow D, Heiati H, Shen WC (2003) J Control Rel 88:369
Wang J, Wu D, Shen WC (2002) Pharm Res 19:609
Ekrami HM, Kennedy AR, Shen WC (1995) FEBS Lett 371:283
Yuan L, Wang J, Shen WC (2005) Pharm Res 22:220
Yuan L, Wang J, Shen WC (2008) J Control Rel 129:11
Wang J, Shen D, Shen WC (1999) Pharm Res 16:1674
Nakato T, Kusuno A, Kakuchi T (2000) J Polym Sci: Part A, Polym Chem 38:117
Tomida M, Nakato T, Matsunami S, Kakuch T (1997) Polymer 38:4733
Nakato T, Tomida M, Suwa M, Morishima Y, Kusuno A, Kakuchi T (2000) Polymer Bull 44:385
Matsubara K, Nakato T, Tomida M (1997) Macromolecular 30:2305
Chang CJ, Swift G (1999) J Macromol Sci Pure Appl Chem 36:963
Kim JH, Son CM, Jeon YS, Choe WS (2011) J Polym Res 18:881
Kang HS, Yang SR, Kim JD, Hang SH, Chang IS (2001) Langmuir 17:7501
Acknowledgments
This work was supported by grants from the National Science Council (NSC 99-2221-E-007-007-MY2), Taiwan, Republic of China.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
(a) H1NMR (500MHz, DMSO-d6) of 6% hexadecylamine-modified PSI (PSI5k- C166%) in DMSO-d6 (b) H1NMR (500MHz , D2O) of 6% hexadecylamine- modified sodium poly(aspartate) (Na-PASP5k-g-C16-PASP5k6%) in D2O. (DOC 302 kb)
Fig. S2
(a) H1NMR (500MHz, DMSO-d6) of 10% hexadecylamine-modified PSI (PSI5k-C1610%) in DMSO-d6 (b) H1NMR (500MHz , D2O) of 10% hexadecylamine - modified sodium poly(aspartate) (Na-PASP5k-g-C16-PASP5k10%) in D2O. (DOC 295 kb)
Fig. S3
(a) H1NMR (500MHz, DMSO-d6) of 15% hexadecylamine-modified PSI (PSI5k-C1615%) in DMSO-d6 (b) H1NMR (500MHz , D2O) of 15% hexadecylamine -modified sodium poly(aspartate) (Na-PASP5k-g-C16-PASP5k15%) in D2O. (DOC 310 kb)
Fig. S4
(a) H1NMR (500MHz, DMSO-d6) of 6% hexadecylamine-modified PSI (PSI8k-C166%) in DMSO-d6 (b) H1NMR (500MHz , D2O) of 6% hexadecylamine- modified sodium poly(aspartate) (Na-PASP8k-g-C16-PASP8k6%) in D2O. (DOC 238 kb)
Fig. S5
(a) H1NMR (500MHz, DMSO-d6) of 10% hexadecylamine-modified PSI (PSI8k-C1610%) in DMSO-d6 (b) H1NMR (500MHz , D2O) of 10% hexadecylamine -modified sodium poly(aspartate) (Na-PASP8k-g-C16-PASP8k10%) in D2O. (DOC 295 kb)
Fig. S6
(a) H1NMR (500MHz, DMSO-d6) of 15% hexadecylamine-modified PSI (PSI8k-C1615%) in DMSO-d6 (b) H1NMR (500MHz , D2O) of 15% hexadecylamine -modified sodium poly(aspartate) (Na-PASP8k-g-C16-PASP8k15%) in D2O. (DOC 310 kb)
Fig. S7
The GPC/SEC chart of Na-PASP5k-g-C16-PASP5k6% with Mw 5417 (DOC 69 kb)
Fig. S8
The GPC/SEC chart of Na-PASP5k-g-C16-PASP5k10% with Mw 6430 (DOC 76 kb)
Fig. S9
The GPC/SEC chart of Na-PASP5k-g-C16-PASP5k15% with Mw 7370 (DOC 74 kb)
Fig. S10
The GPC/SEC chart of Na-PASP8k-g-C16-PASP8k6% with Mw 8757 (DOC 71 kb)
Fig. S11
The GPC/SEC chart of Na-PASP8k-g-C16-PASP8k10% with Mw 10634 (DOC 77 kb)
Fig. S12
The GPC/SEC chart of Na-PASP8k-g-C16-PASP8k15% with Mw 13681 (DOC 75 kb)
Fig.S13
DSC chart of Na-PASP5k-g-C16-PASP5k6% with Tg 52.86 (DOC 138 kb)
Fig. S14
DSC chart of Na-PASP5k-g-C16-PASP5k10% with Tg 47.84 (DOC 68 kb)
Fig. S15
DSC chart of Na-PASP5k-g-C16-PASP5k15% with Tg 48.72 (DOC 121 kb)
Fig. S16
DSC chart of Na-PASP8k-g-C16-PASP8k6% with Tg 77.02 (DOC 51 kb)
Fig. S17
DSC chart of Na-PASP8k-g-C16-PASP8k10% with Tg 57.19°C (DOC 119 kb)
Fig. S18
DSC chart of Na-PASP8k-g-C16-PASP8k15% with Tg 53.58°C (DOC 117 kb)
Fig. S19
I337.5/I335 ratio of Na-PASP5k-g-C16-PASP5k6% with varying concentration (DOC 52 kb)
Fig. S20
I337.5/I335 ratio of Na-PASP5k-g-C16-PAP5k10% with varying concentration (DOC 52 kb)
Fig. S21
I337.5/I335 ratio of Na-PASP5k-g-C16-PASP5k15% with varying concentration (DOC 50 kb)
Fig. S22
I337.5/I335 ratio of Na-PASP8k-g-C16-PASP8k6% with varying concentration (DOC 54 kb)
Fig. S23
I337.5/I335 ratio of Na-PASP8k-g-C16-PASP8k10% with varying concentration (DOC 51 kb)
Fig. S24
I337.5/I335 ratio of Na-PASP8k-g-C16-PASP8k15% with varying concentration (DOC 51 kb)
Rights and permissions
About this article
Cite this article
Hsu, SP., Chu, IM. Design of polyanionic nanocarriers based on modified poly (aspartic acid)s for oral administration: synthesis and characterization. J Polym Res 19, 9913 (2012). https://doi.org/10.1007/s10965-012-9913-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-012-9913-6