Abstract
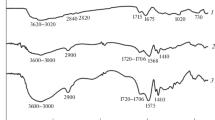
Alcohol-specific superabsorbing gels (super-alcogels) based on non-neutralized acrylic acid (AA, 60–94 mol%) and 2-acrylamido-2-methylpropane sulfonic acid (AMPS) were prepared via solution polymerization in water. Polyethylene glycol dimethacrylate and potassium persulfate were used as crosslinker and initiator, respectively. Characterization of samples was performed using FTIR, 1H-NMR and thermomechanical analyses. Glass transition temperature and modulus of dried samples were found to be directly changed with their AA content. The gels exhibited enormous ability for absorbing and retaining a variety of mono- and poly-hydric alcohols. For example, in lieu of one gram of a typical sample composing 25 mol% AMPS, its absorption capacity was measured to be 53.0 g methanol, 42.1 g ethanol, 12.1 g n-propanol, 3.4 g iso-propanol, 41.2 g ethylene glycol, 20.7 g propylene glycol, 37.8 g 1,3-propanediol and 32.9 g glycerol. The absorbencies were superior to those of a known commercial poly(AA) sample, Carbopol. The alcohol absorbency was improved with increase of AMPS incorporated. It was recognized to be dependant on the alcohol characteristics such as H-bonding ability, OH/C ratio, electronic features (e.g. dielectric constant), steric hindrance of the neighboring groups of the solvent OH group, as well as the solvent viscosity. Normal phase transitions were observed in the gel swelling behavior in the alcohol-water binary mixtures. Rheological measurements of the water-swollen gel showed that more AMPS content resulted in gels with inferior storage modulus. All the empirical observations were discussed based on the related physicochemical principles.









Similar content being viewed by others
References
Zohuriaan-Mehr MJ, Kabiri K (2008) Iran Polym J 17:451
Yavari-Gohar MR, Kabiri K, Zohuriaan-Mehr MJ, Hashemi SA (2010) J Polym Res 17:151
Li A, Wang A, Chen J (2004) J Appl Polym Sci 94:1869
Chen J, Shen J (2000) J Appl Polym Sci 75:1331
Barry BW, Meyer MC (1979) Int J Pharmaceut 2:27
Zhao Q, Liu C (2007) J Appl Polym Sci 105:3458
Kiritoshi Y, Ishihara K (2002) J Biomater Sci Polymer Edn 13:213
Fukunaga Y, Hayashi M, Satoh M (2007) J Polym Sci Part B: Polym Phys 45:1166
Yang H, Song W, Zhuang Y, Deng X (2003) Macromol Biosci 3:400
Kabiri K, Faraji-Dana S, Zohuriaan-Mehr MJ (2005) Polym Adv Technol 16:659
Kabiri K, Mirzadeh H, Zohuriaan-Mehr MJ (2008) J Appl Polym Sci 110:3420
Ramazani-Harandi MJ, Zohuriaan-Mehr MJ, Yousefi AA, Ershad-Langroudi A, Kabiri K (2006) Polym Test 25:470
Omidian H, Zohuriaan-Mehr MJ, Bouhendi H (2003) Int J Polym Mater 52:307
Cascone MG (1997) Polym Int 43:55
Ahn JS, Choi HK, Cho CS (2001) Biomaterials 22:923
Gomez-Carracedo A, Alvarez-Lorenzo C, Gomez-Amoza JL, Concheiro A (2004) Int J Pharmac 274:233
Kabiri K, Mirzadeh H, Zohuriaan-Mehr MJ, Daliri M (2009) Polym Int 2009(58):1252
He Y, Zhu B, Inoue Y (2004) Prog Polym Sci 29:1021
Dong J, Ozaki Y, Nakashima K (1997) Macromolecules 30:1111
Krumova M, López D, Benavente R, Mijangos C, Peren JM (2000) Polymer 41:9265
Gerhartz W, Yamamoto YS, Campbell FT, Pfeffekorn R, Rounsaville JF (eds) (1985) VCH, Weinheim, A1, pp 279–320
Lide DR (2005) CRC handbook of chemistry and physics, 86th edn. Taylor & Francis, Boca Raton, p. 15–13 to 15–22
Gokel GW (2004) Dean’s handbook of organic chemistry, 2nd edn. New York, McGraw-Hill, p 4.57
Nishiyama Y, Satoh M (2000) J Polym Sci Part B Polym Phys 38:2791
Morrison RT, Boyd RN (1983) Organic chemistry, Chap 10, 4th edn. Allyn & Bacon Inc., Boston, 10
Carey FA, Sundberg RJ (2007) Advanced organic chemistry, part A: structure and mechanism, Springer
Rodehed C, Ranby B (1986) Polymer 27:313
Kabiri K, Zohuriaan-Mehr MJ, Mirzadeh H, Kheirabadi M (2010) J Polym Res 17:203
Ono T, Sugimoto T, Shinkai S, Sada K (2007) Nature Mater 6:429
Hu X, Fan J, Yue CY (2001) J Appl Polym Sci 80:2437
Katchalsky A, Eisenberg H (1951) J Polym Sci 6:145
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kabiri, K., Lashani, S., Zohuriaan-Mehr, M.J. et al. Super alcohol-absorbent gels of sulfonic acid-contained poly(acrylic acid). J Polym Res 18, 449–458 (2011). https://doi.org/10.1007/s10965-010-9436-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10965-010-9436-y