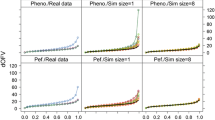
In this paper, the method of estimating bioequivalence, whose main goal is to break the drug concentration curve in the body into components, is considered, implying that this curve is a signal that demonstrates the behavior of the drug. These components are directly related to the main stages of the drug. Denoting the boundaries of these stages, we can, with a minimum of error, compare drugs by the duration and nature of these stages. To isolate the components, methods such as the method of independent components, the window dispersion method, and the study of the variance gamma process will be used.
Similar content being viewed by others
References
J. M. Ennis and D. M. Ennis, “Equivalence hypothesis testing. Food,” Food Qual. Pref., 21, No. 3, 253–256 (2010).
M. A. Dranitsyna, T. V. Zakharova, and R. R. Niyazov, “Properties of the procedure of two unilateral tests for the recognition of bioequivalence of drugs,” Remedium. Mag. Mark. Med. Med. Eq., No. 3, 40–47 (2019).
R. L. Berger and J. C. Hsu, “Rejoinder,” Stat. Sci., 11, No. 4, 315–319 (1996).
M. A. Dranitsyna and T. V. Zakharova, “Investigation of the window variance noise component of the multicomponent signals,” J. Math. Sci., 237, No. 5, 639–645 (2019).
M. A. Dranitsyna and T. V. Zakharova, “Segmentation of nonstationary signals using stochastic characteristic of the window variance,” Inform. Appl., 11, No. 3, 18–26 (2017).
A. Hyvarinen and E. Oja, “Independent component analysis algorithms and application,” Neur. Networks, 411–430 (2000).
Corinne Seng Yue, D. Ozdin, S. Selber-Hnatiw, and M. Ducharme, “Opportunities and challenges related to the implementation of model-based bioequivalence criteria,” Clin. Pharm. Ther., 105, No. 2, 350–362 (2019).
J. Wagner and E. Nelson, “Kinetic analysis of blood levels and urinary excretion in the absorptive phase after single doses of drug,” J. Pharm. Sci., 53, No. 11, 1392–1403 (1964).
K. Yamaoka, T. Nakagawa, and T. Uno, “Statistical moments in pharmacokinetics,” J. Pharmacokinet. Biopharm., 6, No. 6, 547–558 (1978).
Author information
Authors and Affiliations
Corresponding author
Additional information
Proceedings of the XXXV International Seminar on Stability Problems for Stochastic Models, Perm, Russia, September 24–28, 2018. Part I.
Rights and permissions
About this article
Cite this article
Slivkina, A.V., Zakharova, T.V. & Dranitsyna, M. The Application of the ICA Method and Window Dispersion in the Study of Bioequivalence of Drugs. J Math Sci 246, 540–547 (2020). https://doi.org/10.1007/s10958-020-04758-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10958-020-04758-5