Abstract
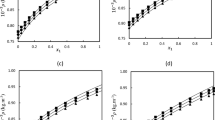
The experimental measurement of density and enthalpy of mixture for two binary liquid mixtures of n-tridecane or n-tetradecane with decalin was reported in this paper. The measurements were conducted at a temperature range of 293.15–323.15 K and at 303.15 K using calorimeter C80. The mixtures were analyzed at various proportions, including the entire composition range and dilute solutions. The excess molar volume (VE) and excess molar enthalpy (HE) of mixtures were calculated and fitted using the Redlich–Kister equation. The paper observed the expansion phenomenon for the VE at all temperatures, including over the entire composition range and dilute solutions. Additionally, the HE exhibited endothermic behavior at the studied temperature range and composition range. The Prigogine–Flory–Patterson (PFP) theory was utilized to predict both thermodynamic properties, namely the VE and HE. The results obtained using the PFP theory were compared with those obtained using the Treszczanowicz and Benson association (TB) model for VE and with the NRTL, Wilson, and Flory models for HE. The PFP model, which employed a single-fitted parameter to describe VE, demonstrated satisfactory performance in predicting VE. Conversely, the Treszczanowicz and Benson association (TB) model yielded relatively poor results in fitting VE. However, the NRTL, Wilson, PFP, and Flory models exhibited good performance in predicting HE.









Similar content being viewed by others
References
Pan, L.: Combustion properties of fuels and methods to improve them. In: Zou, J.-J., Zhang, X., Pan, L. (eds.) High-Energy-Density Fuels for Advanced Propulsion: Design and Synthesis, pp. 437–473. Wiley, Hoboken (2020). https://doi.org/10.1002/9783527823789.ch9
Pires, A.P., Han, Y., Kramlich, J., Garcia-Perez, M.: Chemical composition and fuel properties of alternative jet fuels. BioResources 13(2), 2632–2657 (2018). https://doi.org/10.15376/biores.13.2.2632-2657
Braun-Unkhoff, M., Kathrotia, T., Rauch, B., Riedel, U.: About the interaction between composition and performance of alternative jet fuels. CEAS Aeronaut. J. 7, 83–94 (2016). https://doi.org/10.1007/s13272-015-0178-8
Eller, Z., Varga, Z., Hancsók, J.: Advanced production process of jet fuel components from technical grade coconut oil with special hydrocracking. Fuel 182, 713–720 (2016). https://doi.org/10.1016/j.fuel.2016.06.055
Meeks, E., Naik, C.V., Puduppakkam, K.V., Modak, A., Egolfopoulos, F.N., Tsotsis, T., Westbrook, C.K.: Experimental and modeling studies of the combustion characteristics of conventional and alternative jet fuels. Final Report (2011).
Outcalt, S., Laesecke, A., Freund, M.B.: Density and speed of sound measurements of Jet A and S-8 aviation turbine fuels. Energy Fuels. 23(3), 1626–1633 (2009). https://doi.org/10.1021/ef800888q
Zehioua, R., Coquelet, C., Soo, C.B., Richon, D., Meniai, A.H.: Experimental and predicted excess molar enthalpies of some working pairs for absorption cycles. Thermochim. Acta. 495(1–2), 72–80 (2009). https://doi.org/10.1016/j.tca.2009.06.004
Letcher, T., Spiteri, W., Scoones, B.: Excess enthalpies of decahydronaphthalene in cycloalkanes and in n-alkanes at two temperatures. J. Sol. Chem. 11, 423–434 (1982). https://doi.org/10.1007/BF00649041
Yu, L., Wu, Z., Qiu, Y., Qian, Y., Mao, Y., Lu, X.: Ignition delay times of decalin over low-to-intermediate temperature ranges: rapid compression machine measurement and modeling study. Combust. Flame. 196, 160–173 (2018). https://doi.org/10.1016/j.combustflame.2018.06.014
Yao, C., Mi, J., Jiang, P., Ye, C., Dai, Y., Guo, Y., Fang, W.: Densities and viscosities of the ternary mixtures of decalin (1)+ n-hexadecane (2)+ 1-butanol (3) and corresponding binary systems. J. Chem. Eng. Data. 68(4), 869–880 (2023). https://doi.org/10.1021/acs.jced.3c00003
Huber, M.L., Lemmon, E.W., Diky, V., Smith, B.L., Bruno, T.J.: Chemically authentic surrogate mixture model for the thermophysical properties of a coal-derived liquid fuel. Energy Fuels. 22(5), 3249–3257 (2008). https://doi.org/10.1021/ef800314b
Touazi, A.A., Didaoui, S., Khimeche, K., Benziane, M.: Thermophysical properties investigation of high-density jet fuel with alcohols additives. Int. J. Thermophys. 41(9), 130 (2020). https://doi.org/10.1007/s10765-020-02713-9
Touazi, A.A., Didaoui, S., Khimeche, K., Benziane, M.: Measurement and prediction of thermophysical properties of binary mixtures of dicyclopentadiene with methylcyclohexane, toluene, and p-xylene. Thermochim. Acta. 685, 178536 (2020). https://doi.org/10.1016/j.tca.2020.178536
Touazi, A.A., Khellili, E., Didaoui, S., Khimeche, K., Benziane, M.: Measurement and correlation of isobaric densities and viscosities for endo-dicyclopentadiene with hydrocarbons (C6–C8) at different temperatures. J. Thermal Anal. Calorimetry. 134, 1223–1242 (2018). https://doi.org/10.1007/s10973-018-7583-2
Touazi, A.A., Didaoui, S., Khimeche, K., Rial, M., Moulai, S.A., Benziane, M.: Experimental and theoretical studies of volumetric and viscous properties of n-tetradecane with isomeric pentanols. J. Mol. Liquids. 327, 114860 (2021). https://doi.org/10.1016/j.molliq.2020.114860
Maskey, S., Morrow, B.H., Gustafson, M.Z., Prak, D.J.L., Mikulski, P.T., Harrison, J.A.: Systematic examination of the links between composition and physical properties in surrogate fuel mixtures using molecular dynamics. Fuel. 261, 116247 (2020). https://doi.org/10.1016/j.fuel.2019.116247
Dohnal, V., Řehák, K.: Determination of infinite dilution partial molar excess enthalpies and volumes for some ionic liquid precursors in water and methanol using tandem flow mixing calorimetry and vibrating-tube densimetry. J. Chem. Eng. Data 56(7), 3047–3052 (2011). https://doi.org/10.1021/je200093f
Trampe, D.M., Eckert, A.C.: Calorimetric measurement of partial molar excess enthalpies at infinite dilution. J. Chem. Eng. Data. 36(1), 112–118 (1991). https://doi.org/10.1021/je00001a033
Yu, J., Eser, S.: Thermal decomposition of jet fuel model compounds under near-critical and supercritical conditions. 2. Decalin and tetralin. Ind. Eng. Chem. Res. 37(12), 4601–4608 (1998). https://doi.org/10.1021/ie980302y
Amara, A.B., Kaoubi, S., Starck, L.: Toward an optimal formulation of alternative jet fuels: Enhanced oxidation and thermal stability by the addition of cyclic molecules. Fuel. 173, 98–105 (2016). https://doi.org/10.1016/j.fuel.2016.01.040
Wang, R., Li, G., Tang, H., Wang, A., Xu, G., Cong, Y., Li, N.: Synthesis of decaline-type thermal-stable jet fuel additives with cycloketones. ACS Sustain. Chem. Eng. 7(20), 17354–17361 (2019). https://doi.org/10.1021/acssuschemeng.9b04288
Muldoon, J.A., Harvey, B.G.: Bio-based cycloalkanes: the missing link to high-performance sustainable jet fuels. ChemSusChem. 13(22), 5777–5807 (2020). https://doi.org/10.1002/cssc.202001641
Matos, J.S., Trenzado, J.L., Caro, M.N., Romano, E., Pérez, E.: Volumetric study of (an aliphatic methyl ester + heptane or nonane) at the temperature 298.15 K. J. Chem. Thermodyn. 26(8), 857–862 (1994). https://doi.org/10.1006/jcht.1994.1102
Zhang, R., Chen, J., Mi, J.: Excess molar enthalpies for binary mixtures of different amines with water. J. Chem. Thermodyn. 89, 16–21 (2015). https://doi.org/10.1016/j.jct.2015.04.030
Abe, A., Flory, P.: The thermodynamic properties of mixtures of small, nonpolar molecules. J. Am. Chem. Soc. 87(9), 1838–1846 (1965). https://doi.org/10.1021/ja01087a003
Redlich, O., Kister, A.: Algebraic representation of thermodynamic properties and the classification of solutions. Ind. Eng. Chem. 40(2), 345–348 (1948). https://doi.org/10.1021/ie50458a036
Zarei, H., Bohloor, F., Omidi, A.: Excess molar enthalpies of ethane-1,2-diamine plus primary and secondary alkanols (C1–C4) and correlation with Redlich-Kister, Wilson, NRTL and UNIQUAC models at T = 298 K. J. Chem. Thermodyn. (2017). https://doi.org/10.1016/j.jct.2016.12.036
Wilson, G.M.: Vapor-liquid equilibrium. XI. A new expression for the excess free energy of mixing. J. Am. Chem. Soc. 86(2), 127–130 (1964). https://doi.org/10.1021/ja01056a002
Prigogine, I., Bellemans, A., Mathot, V.: The molecular theory of solutions, NorthHolland. Amsterdam (1957). https://doi.org/10.11316/butsuri1946.12.11.534
Flory, P., Orwoll, R., Vrij, A.: Statistical thermodynamics of chain molecule liquids. I. An equation of state for normal paraffin hydrocarbons. J. Am. Chem. Soc. 86(17), 3507–3514 (1964). https://doi.org/10.1021/ja01071a023
Zhang, C., Wang, R., Hui, X., Xue, X., Lin, Y., Sung, C.J.: Nonlinear threshold sooting index prediction method for surrogate formulation emulating sooting characteristics: a case study using RP-3 jet fuels. Energy Fuels. 34(8), 9990–9999 (2020). https://doi.org/10.1021/acs.energyfuels.0c00921
Letinski, D.J., Parkerton, T.F., Redman, A.D., Connelly, M.J., Peterson, B.: Water solubility of selected C9–C18 alkanes using a slow-stir technique: comparison to structure–property models. Chemosphere. 150, 416–423 (2016). https://doi.org/10.1016/j.chemosphere.2015.12.038
Alvarez, V., Mattedi, S., Aznar, M.: Density, refraction index, and vapor–liquid equilibria of n-methyl-2-hydroxyethylammonium hexanoate plus (methyl acetate, ethyl acetate, or propyl acetate) at several temperatures. Ind. Eng. Chem. Res. 51(44), 14543–14554 (2012). https://doi.org/10.1021/ie302343v
Kim, S.H., Roth, M.: Enthalpies of dilution and excess molar enthalpies of an aqueous solution of sulfuric acid. J. Chem. Eng. Data 46(1), 138–143 (2001). https://doi.org/10.1021/je0000221
González, B., Dominguez, A., Tojo, J., Cores, R.: Dynamic viscosities of 2-pentanol with alkanes (Octane, Decane, and Dodecane) at three temperatures T (293.15, 298.15, and 303.15) K. New UNIFAC−VISCO interaction parameters. J. Chem. Eng. Data 49(5), 1225–1230 (2004). https://doi.org/10.1021/je034208m
Larsen, B.L., Rasmussen, P., Fredenslund, A.: A modified UNIFAC group-contribution model for prediction of phase equilibria and heats of mixing. Ind. Eng. Chem. Res. 26(11), 2274–2286 (1987). https://doi.org/10.1021/ie00071a018
Mohammadi, L., Omrani, A.: Density, refractive index, and excess properties of sulfolane and alkanediols binary mixtures at different temperatures. J. Therm. Anal. Calorim. 131, 1527–1543 (2018). https://doi.org/10.1007/s10973-017-6702-9
Patterson, D., Delmas, G.: Corresponding states theories and liquid models. Discuss. Faraday Soc. 49, 98–105 (1970). https://doi.org/10.1039/DF9704900098
Su, C., Zhu, C., Lai, T., Wang, T., Liu, X., He, M.: Temperature and pressure dependence of densities and viscosities for binary mixtures of methyl decanoate plus n-heptane. Thermochim. Acta. 670, 211–218 (2018). https://doi.org/10.1016/j.tca.2018.10.018
Gahlyan, S., Verma, S., Rani, M., Maken, S.: Excess molar enthalpy of oxygenate+ hydrocarbon mixtures: application of Flory–Treszczanowicz–Benson model and graph theoretical approach. J. Mol. Liquids. 258, 142–146 (2018). https://doi.org/10.1016/j.molliq.2018.03.003
Bhardwaj, U., Maken, S., Singh, K.: Excess molar enthalpies of 2-methylpropan-1-ol with aromatic hydrocarbons at 308.15 K in terms of an association model with a Flory contribution term. Fluid Phase Equilibria. 142(1–2), 205–213 (1998). https://doi.org/10.1016/S0378-3812(97)00227-6
Gahlyan, S., Rani, M., Maken, S., Kwon, H., Tak, K., Moon, I.: Modeling of thermodynamic properties of an oxygenate+ aromatic hydrocarbon: excess molar enthalpy. J. Ind. Eng. Chem. 23, 299–306 (2015). https://doi.org/10.1016/j.jiec.2014.08.032
Hu-Gang, Z., Zhi-Hua, L., Yi-Ling, T., Yuan, X., Liang, Y.: Molar volume, thermal expansivity and isothermal compressibility of trans-decahydronaphthalene up to 200MPa and 446K. Chin. Phys. 14(12), 2433 (2005). https://doi.org/10.1088/1009-1963/14/12/011
Aicart, E., Tardajos, G., Peña, M.D.: Isothermal compressibility of cyclohexane+ n-tridecane and+ n-pentadecane at 298.15, 308.15, 318.15, and 333.15 K. J. Chem. Thermodyn. 13(8), 783–788 (1981). https://doi.org/10.1016/0021-9614(81)90067-7
Lei, Q., Dai, Y., Wu, P., Fang, W., Guo, Y.: densities and viscosities for the ternary system of isopropylcyclohexane (1)+ n-tridecane (2)+ n-butanol (3) and corresponding binaries at T= 293.15 to 333.15 K. J. Chem. Eng. Data. 65(8), 3977–3987 (2020). https://doi.org/10.1021/acs.jced.0c00309
Camin, D.L., Rossini, F.D.: Physical Properties of Fourteen API Research Hydrocarbons, C9 to C15. J. Phys. Chem 59(11), 1173–1179 (1955). https://doi.org/10.1021/j150533a014
Luning Prak, D.J., Lee, B.G., Cowart, J.S., Trulove, P.C.: Density, viscosity, speed of sound, bulk modulus, surface tension, and flash point of binary mixtures of butylbenzene+ linear alkanes (n-decane, n-dodecane, n-tetradecane, n-hexadecane, or n-heptadecane) at 0.1 MPa. J. Chem. Eng. Data. 62(1), 169–187 (2017). https://doi.org/10.1021/acs.jced.6b00542
Chi, H., Li, G., Guo, Y., Xu, L., Fang, W.: Excess molar volume along with viscosity, flash point, and refractive index for binary mixtures of cis-decalin or trans-decalin with C9 to C11 n-alkanes. J. Chem. Eng. Data. 58(8), 2224–2232 (2013). https://doi.org/10.1021/acs.jced.6b00542
Shiohama, Y., Ogawa, H., Murakami, S., Fujihara, I.: Molar excess enthalpies of cis-decalin+ benzene,+ toluene,+ isooctane and+ heptane at 298.15 K. Fluid Phase Equilibria 32(3), 249–260 (1987). https://doi.org/10.1016/0378-3812(87)85057-4
Miyake, Y., Baylaucq, A., Zéberg-Mikkelsen, C.K., Galliéro, G., Ushiki, H., Boned, C.: Stereoisomeric effects on volumetric properties under pressure for the system cis- + trans-decalin. Fluid Phase Equilibria. 252, 79–87 (2007). https://doi.org/10.1016/j.fluid.2006.12.011
Pena, M.D., Tardajos, G.: Isothermal compressibility of cyclohexane + n-tridecane and + n-pentadecane at 298.15, 308.15, 318.15, and 333.15 K. M-786. J. Chem. Thermodyn. 13, 783–788 (1981). https://doi.org/10.1016/0021-9614(81)90067-7
Pena, M.D., Tardajos, G.: Isothermal compressibility of n-alkanes and benzene. J. Chem. Thermodyn. 10, 19–24 (1978). https://doi.org/10.1016/0021-9614(78)90144-1
Funding
This work was supported by the Ecole Supérieure du Matériel.
Author information
Authors and Affiliations
Contributions
Ahmed Amin TOUAZI and Abdelnour Boussadia wrote the main manuscript. Ahmed Amin TOUAZI, Abdelnour Boussadia, and Chalghoum Fateh prepared all figures and Tales. Ahmed Amin TOUAZI, Saéda Didaoui, Noureddine Nasrallah, and Mokhtar Benziane reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
No potential conflict of interest was reported by the author.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Touazi, A.A., Boussaadia, A., Didaoui, S. et al. Measurement and Modeling of Excess Molar Volume and Excess Enthalpy of n-Tridecane or n-Tetradecane with Decalin by Application of PFP Theory. J Solution Chem (2024). https://doi.org/10.1007/s10953-024-01374-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10953-024-01374-8