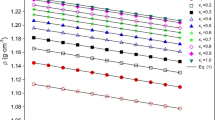
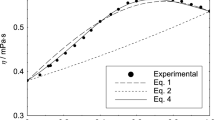
Abstract
Density and viscosity measurements were carried out at 25 °C (298.15 K) on six binary di(or tri)glyme/organic carbonate (dimethylcarbonate, ethylene carbonate or propylene carbonate) systems and on multicomponent systems containing di(or tri)glyme/ organic carbonate mixtures. The effect of the organic carbonate composition on the excess weight volume (VwE), the deviation viscosity (\(\Delta \eta\)), the partial weight and apparent weight volumes of glymes is interpreted in terms of competitive interactions and/or of differences in size or shape between glymes and organic carbonates. In addition, a model, based on viscosity data, is developed for the first time to determine the hydrodynamic volumes of glymes. The comparison of hydrodynamic volumes with Van der Waals volumes makes it possible to highlight and quantify the possible interactions between glymes and pure organic carbonates, and the association of triglyme molecules in organic carbonate EC/PC mixtures. The effect of LiPF6 and NaPF6 salts at 1 mol⋅L−1 in the glyme/organic carbonate mixtures appears to significantly reduce the volumes of glymes. By this, the strong interactions between salts and glymes are shown.









Similar content being viewed by others
References
Engelbrecht, L. de V., Mocci, F., Wang, Y., Perepelytsya, S., Vasiliu, T., Laaksonen, A.: Molecular perspective on solutions and liquid mixtures from modelling and experiment. Springer Proc. Phys. 266, 53–84 (2022)
Jouyban, A., Acree, W.E.: A single model to represent physico-chemical properties of liquid mixtures at various temperatures. J. Mol. Liq. 323, 115054 (2021). https://doi.org/10.1016/j.molliq.2020.115054
Lenahan, F.D., Zhai, Z., Kankanamge, C.J., Klein, T., Fröba, A.P.: Viscosity and interfacial tension of ternary mixtures consisting of linear alkanes, alcohols, and/or dissolved gases using surface light scattering and equilibrium molecular dynamics simulations. Int. J. Thermophys. 43, 116 (2022). https://doi.org/10.1007/s10765-022-03040-x
Devi, N.G., Srinivasa Rao, N.V.N.B., Ramachandran, D., Nagalakshmi, V., Sunila Rani, P.: Viscometric study on binary liquid mixtures of propiophenone with aniline and N-alkyl substituted anilines, at 30315 to 31815 K. Rasayan J. Chem. 15, 292–301 (2022). https://doi.org/10.31788/RJC.2022.1516663
Saini, A., Verma, S., Harshavardhan, A., Dey, R.: Two new models for viscosity prediction of binary, ternary and higher order liquid mixtures. RSC Adv. 6, 113657–113662 (2016). https://doi.org/10.1039/C6RA24532C
Tang, S., Zhao, H.: Glymes as versatile solvents for chemical reactions and processes: from the laboratory to industry. RSC Adv. 4, 11251–11287 (2014). https://doi.org/10.1039/C3RA47191H
No, S.-Y.: Application of bio-oils from lignocellulosic biomass to transportation, heat and power generation-a review. Renew. Sustain. Energy Rev. 40, 1108–1125 (2014). https://doi.org/10.1016/j.rser.2014.07.127
Devi, B.K., Naraparaju, S., Soujanya, C., Gupta, S.D.: Green chemistry and green solvents: an overview. Curr. Green Chem. 7, 314–325 (2020). https://doi.org/10.2174/2213346107999200709132815
Shaikh, A.-A.G., Sivaram, S.: Organic carbonates. Chem. Rev. 96, 951–976 (1996)
Kandpal, C., Pandey, J.D., Dey, R., Kumar Singh, A., Kumar, V., Singh.: Comparative study of viscosity, diffusion coefficient, thermal conductivity and Gibbs free energy for binary liquid mixtures at varying temperatures. J. Mol. Liq. 333, 115858 (2021). https://doi.org/10.1016/j.molliq.2021.115858
González, J.A., Martínez, F.J., Sanz, L.F., Hevia, F., de la Fuente, I.G., Cobos, J.C.: Volumetric and viscosimetric measurements for methanol + CH3-O-(CH2CH2O)n-CH3 (n = 2, 3, 4) mixtures at (293.15–303.15) K and atmospheric pressure: application of the ERAS model. J. Solut. Chem. 49, 332–352 (2020).https://doi.org/10.1007/s10953-020-00964-6
Rivas, M.A., Iglesias, T.P.: On permittivity and density of the systems triglyme + (dimethyl or diethyl carbonate) and formulation of ∆ε in terms of volume or mole fraction. J. Chem. Thermodyn. 40, 1120–1130 (2008)
Vani Latha, S., Little Flower, G., Rayapa Reddy, K., Nageswara Rao, C.V., Ratnakar, A.: Densities, ultrasonic velocities, excess properties and IR spectra of binary liquid mixtures of organic esters (ethyl lactate, some organic carbonates). J. Solut. Chem. 46, 305–330 (2017). https://doi.org/10.1007/s10953-016-0567-6
Chaudoy, V., Ghamouss, F., Jacquemin, J., Houdbert, J.C., Tran-Van, F.: On the performances of ionic liquid-based electrolytes for Li-NMC batteries. J. Solut. Chem. 44, 769–789 (2015). https://doi.org/10.1007/s10953-015-0315-3
Bodenes, L., Darwiche, A., Monconduit, L., Martinez, H.: The solid electrolyte interphase a key parameter of the high performance of Sb in sodium-ion batteries: comparative X-ray photoelectron spectroscopy study of Sb/Na-ion and Sb/Li-ion batteries. J. Power Sources 273, 14–24 (2015). https://doi.org/10.1016/j.jpowsour.2014.09.037
Eshetu, G.G., Grugeon, S., Kim, H., Jeong, S., Wu, L., Gachot, G., Laruelle, S., Armand, M., Passerini, S.: ChemSusChem 9, 462–471 (2016). https://doi.org/10.1002/cssc.201501605
Spieweck, F., Bettin, H.: Review: Solid and liquid density determination. Tech. Mess. 59, 285–292 (1992). https://doi.org/10.1524/teme.1992.59.78.285
Sinclair, C.D., Vincent, C.A.: Derivation of partial molal volumes in binary systems. J. Chem. Soc. Faraday Trans. I 70, 1926–1933 (1974). https://doi.org/10.1039/F19747001926
Einstein, A.: Investigations on the theory of the Brownian movement. In: Fürth, R., Cowper, A.D. (eds.) Translator. Dover, New York (1956)
McPhie, M.G., Daivis, P.J., Snook, I.K.: Viscosity of a binary mixture: approach to the hydrodynamic limit. Phys. Rev. E 74, 031201 (2006). https://doi.org/10.1103/PhysRevE.74.031201
Wajnryb, E., Dahler, J.S.: The viscosity of a moderately dense, polydisperse suspension of spherical particles. Physica A 253, 77–104 (1998). https://doi.org/10.1016/S0378-4371(97)00682-1
Batchelor, G.K.: The effect of Brownian motion on the bulk stress in a suspension of spherical particles. J. Fluid Mech. 83, 97–117 (1977). https://doi.org/10.1017/S0022112077001062
Haruki, S., Sukenaga, S., Saito, N., Nakashima, K.: Viscosity estimation of spherical particles dispersed suspension. High Tem. Mater. Proc. 30, 405–409 (2011)
Mader, H.M., Llewellin, E.W., Mueller, S.P.: The rheology of two-phase magmas: a review and analysis. J. Volcanol. Geotherm. Res. 257, 135–158 (2013)
Fort, R.J., Moore, W.R.: Viscosities of binary liquid mixtures. Trans. Faraday Soc. 62, 1112–1119 (1966). https://doi.org/10.1039/TF9666201112
Grunberg, L., Nissan, A.H.: Mixture law for viscosity. Nature 164, 799–800 (1949)
Katti, P.K., Chaudhri, M.M.: Viscosities of binary mixtures of benzyl acetate with dioxane, aniline, and m-cresol. J. Chem. Eng. Data 9, 442–443 (1964). https://doi.org/10.1021/je60022a047
Heric, E.L., Brewer, J.G.: Viscosity of some binary liquid nonelectrolyte mixtures. J. Chem. Eng. Data 12, 574–583 (1967). https://doi.org/10.1021/je60035a028
Hind, R.K., McLaughlin, E., Ubbelohde, A.R.: Structure and viscosity of liquids camphor + pyrene mixtures. Trans. Faraday Soc. 56, 328–330 (1960). https://doi.org/10.1039/TF9605600328
Tamura, M., Kurata, M.: On the viscosity of binary mixture of liquids. Bull. Chem. Soc. Jpn. 25, 32–38 (1952). https://doi.org/10.1246/bcsj.25.32
Novak, L.T.: Relationship between the intrinsic viscosity and Eyring-NRTL viscosity model parameters. Ind. Eng. Chem. Res. 43, 2602–2604 (2004). https://doi.org/10.1021/ie040010z
Sadeghi, R.: Segment-based Eyring-Wilson viscosity model for polymer solutions. J. Chem. Thermodyn. 37, 445–448 (2005). https://doi.org/10.1016/j.jct.2004.10.009
Horita, K., Sawatari, N., Yoshizaki, T., Einaga, Y., Yamakawa, H.: Excluded-volume effects on the transport coefficients of oligo- and poly(dimethylsiloxane)s in dilute solution. Macromolecules 28, 4455–4463 (1995). https://doi.org/10.1021/ma00117a014
Huglin, M.B., Sokro, M.B.: Characterization of oligo- poly(dimethylsiloxanes) and their solutions in toluene. Polymer 21, 651–658 (1980). https://doi.org/10.1016/0032-3861(80)90323-7
Dodgson, K., Semlyen, J.A.: Studies of cyclic and linear poly(dimethylsiloxanes): 1 Limiting viscosity number-molecular weight relationships. Polymer 18, 1265–1268 (1977). https://doi.org/10.1016/0032-3861(77)90291-9
Barry, A.J.: Viscometric investigation of dimethylsiloxane polymers. J. Appl. Phys. 17, 1020–1024 (1946). https://doi.org/10.1063/1.1707670
Mark, J.E.: Polymer data handbook. Oxford University Press, New York (1999)
Bates, O.K.: Thermal conductivity of liquid silicones. Ind. Eng. Chem. 41, 1966–1968 (1949)
Ancherbak, S., Yasnou, V., Mialdun, A., Shevtsova, V.: Coexistence curve, density, and viscosity for the binary system of perfluorohexane + silicone oil. J. Chem. Eng. Data 63, 3008–3017 (2018). https://doi.org/10.1021/acs.jced.8b00278
Sakai, K., Iijima, S., Ikeda, R., Endo, T., Yamazaki, T., Yamashita, Y., Natsuisaka, M., Sakai, H., Abe, M., Sakamoto, K.: Water-in-oil emulsions prepared by peptide-silicone hybrid polymers as active interfacial modifier: effects of silicone oil species on dispersion stability of emulsions. J. Oleo Sci. 62, 505–511 (2013)
Di Nicola, G., Pierantozzi, M., Tomassetti, S., Coccia, G.: Surface tension calculation from liquid viscosity data of silanes. Fluid Phase Equil. 463, 11–17 (2018). https://doi.org/10.1016/j.fluid.2018.01.005
Chopra, D., Kontopoulou, M., Vlassopoulos, D., Hatzikiriakos, S.G.: Interrelations between rheology and phase behaviour in partially miscible blends: the case of polydimethylsiloxane/polyethylmethylsiloxane (PDMS/PEMS). Can. J. Chem. Eng. 80, 1057–1064 (2002). https://doi.org/10.1002/cjce.5450800607
Hunter, M.J., Warrick, E.L., Hyde, J.F., Currie, C.C.: Organosilicon polymers. II. The open chain dimethylsiloxanes with trimethylsiloxy end groups. J. Am. Chem. Soc. 68, 2284–2290 (1946)
Pal, A., Kumar, A.: Excess molar volumes, viscosities, and refractive indices of diethylene glycol dimethyl ether with dimethyl carbonate, diethyl carbonate, and propylene carbonate at (298.15, 308.15, and 318.15) K. J. Chem. Eng. Data 43, 143–147 (1998). https://doi.org/10.1021/je9701902
Pal, A., Dass, G., Kumar, A.: Excess molar volumes, viscosities, and refractive indices of triethylene glycol dimethyl ether with dimethyl carbonate, diethyl carbonate, and propylene carbonate at 298.15 K. J. Chem. Eng. Data 43, 738–741 (1998). https://doi.org/10.1021/je980016t
Xu, K.: Nonaqueous liquid electrolytes for lithium-based rechargeable batteries. Chem. Rev. 104, 4303–4417 (2004). https://doi.org/10.1021/cr030203g
Reis, J.C.R., Iglesias, T.P.: Kirkwood correlation factors in liquid mixtures from an extended Onsager–Kirkwood–Fro ̈hlich equation. Phys. Chem. Chem. Phys. 13, 10670–10680 (2011). https://doi.org/10.1039/c1cp20142e
Conesa, A., Shen, S., Coronas, A.: Liquid densities, kinematic viscosities, and heat capacities of some ethylene glycol dimethyl ethers at temperatures from 283.15 to 423.15 K. Int. J. Thermophys. 19, 1343–1358 (1998)
Solvents, O.: Physical properties and methods of purification, 4th edn. Wiley, New York (1986)
Zhao, Y.H., Abraham, M.H., Zissimos, A.M.: Fast calculation of van der Waals volume as a sum of atomic and bond contributions and its application to drug compounds. J. Org. Chem. 68, 7368–7373 (2003). https://doi.org/10.1021/jo034808o
Yoshida, K., Tsuchiya, M., Tachikawa, N., Dokko, K., Watanabe, M.: Change from glyme solutions to quasi-ionic liquids for binary mixtures consisting of lithium bis(trifluoromethanesulfonyl)amide and glymes. J. Phys. Chem. C 115(37), 18384–18394 (2011). https://doi.org/10.1021/jp206881t
Saito, M., Yamada, S., Ishikawa, T., Otsuka, H., Ito, K., Kubo, Y.: Factors influencing fast ion transport in glyme-based electrolytes for rechargeable lithium–air batteries. RSC Adv. 7, 49031–49040 (2017). https://doi.org/10.1039/c7ra07501d
Matsuda, Y., Morita, M., Tachihara, F.: Conductivity of lithium salts in the mixed systems of high permittivity solvents and low viscosity solvents. Bull. Chem. Soc. Jpn. 59, 1967–1973 (1986)
Borodin, O., Smith, G.D.: Development of many-body polarizable force fields for Li-battery components: 1. Ether, alkane, and carbonate-based solvents. J. Phys. Chem. B 110, 6293–6299 (2006). https://doi.org/10.1021/jp055079e
Gomez-Camer, J.L., Acebedo, B., Ortiz-Vitoriano, N., Monterrubio, I., Galceran, M., Rojo, T.: Unravelling the impact of electrolyte nature on Sn4P3/C negative electrodes for Na-ion batteries. J. Mater. Chem. A 7, 18434–18441 (2019). https://doi.org/10.1039/c9ta04288a
Ponrouch, A., Marchante, E., Courty, M., Tarascon, J.-M., Palacin, M.R.: In search of an optimized electrolyte for Na-ion batteries. Energy Environ. Sci. 5, 8572–8583 (2012). https://doi.org/10.1039/c2ee22258b
Kim, H., Hong, J., Park, Y.-U., Kim, J., Hwang, I., Kang, K.: Sodium storage behavior in natural graphite using ether-based electrolyte systems. Adv. Func. Mater. 25, 534–541 (2015). https://doi.org/10.1002/adfm.201402984
Funding
No funds, grants, or other support was received for conducting this study.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study involved in the present work. Material preparation, data collection and analysis were performed by CT, CD and RN. The first draft of the manuscript was written by CD and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tachouaft, C., Damas, C. & Naejus, R. Effect of Organic Carbonate Solvent Composition on the Volumetric and Viscometric Behavior of Linear Ethers. J Solution Chem 52, 1232–1254 (2023). https://doi.org/10.1007/s10953-023-01312-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-023-01312-0