Abstract
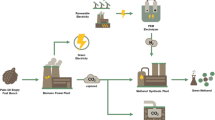
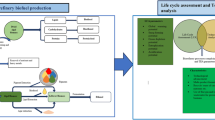
The growing need for food and energy around the world, as well as the impending water stress due to global warming exacerbated by increasing anthropogenic greenhouse gas emissions, makes sound solutions for sustainable development essential. An alternative is the concept of polygeneration which, based on process intensification, makes it possible to simultaneously obtain several products, chemical or energy, from a single source, preferably renewable. This work aims to highlight the synergy between thermodynamics and chemical reaction in a polygeneration model “bioproducts-bioenergy-water (BBW)” whose two building blocks are illustrated, i.e., (i) solar desalination with power and hydrogen production as well as brine valorization and (ii) production of bioenergy (biodiesel-2G) and bioproducts (biolubricants) based on green circular economy. It is shown that scientific and technological building blocks where thermodynamics and chemical reaction successfully operate in synergy for enhancing process intensification are available to implement the triptych “BBW” that would secure the supply of vital human needs and thus preserve global stability.












Similar content being viewed by others
Abbreviations
- 2E1H:
-
2-Ethyl-1-hexanol
- 2G:
-
Second generation
- 3G:
-
Third generation
- 3E :
-
Eco-design, eco-material, eco-energy
- BioEt:
-
Bioethanol
- CPA:
-
Cubic-Plus-Association
- CSP:
-
Concentrating solar power
- EB:
-
Ethyl butanoate
- EoS:
-
Equation of state
- GCA:
-
Group contribution with association
- GDP:
-
Gross domestic product
- HTF:
-
Heat transfer fluid
- IP:
-
Isopentane, also designated R601a in the refrigerant list by IUPAC
- IMSO:
-
Indian mustard seed oil
- IMSOEEs:
-
Indian mustard seed oil ethyl esters
- IMSO2E1HEs:
-
Indian mustard seed oil 2-ethyl-1-hexanol esters
- LTMED:
-
Low-temperature multi-effect distillation
- ORC:
-
Organic Rankine cycle
- PTC:
-
Parabolic trough collector
- RO:
-
Reverse osmosis
- RC:
-
Rankine cycle
- Sc:
-
Supercritical
- TG:
-
Triglycerides
- UMR-PR:
-
Universal Mixing Rule-Peng-Robinson
- UNIFAC:
-
UNIversal Functional Activity Coefficient
- VLE:
-
Vapor–liquid equilibria
- \(AAD_{i} (x)\) :
-
Average absolute deviation between experimental and calculated property x of component i
- \(AAD_{k} (x)\) :
-
Average absolute deviation between experimental and calculated property x of dataset k
- \(\dot{E}x_{d}\) :
-
Exergy destruction rate occurring inside the considered system
- \(\overline{E}x_{k}\) :
-
Mass exergy of the fluid flowing in stream k
- \(\overline{H}_{k}\) :
-
Mass enthalpy of the fluid flowing in stream k
- \(\dot{m}_{k}\) :
-
Mass flowrate of the fluid flowing in stream k
- P :
-
Pressure
- \(\dot{Q}_{k}\) :
-
Thermal power provided to (or generated by) unit k
- R :
-
Ideal gas constant
- R c R :
-
Recovery ratio
- \(\overline{S}_{k}\) :
-
Mass entropy of the fluid flowing in stream k
- SMR :
-
Stream mass ratio
- T :
-
Temperature
- \(T_{ref}\) :
-
Reference temperature (293.15 K)
- \(\dot{W}_{k}\) :
-
Mechanical power provided to (or generated by) unit k
- x :
-
Liquid mole fraction
- y :
-
Vapor mole fraction
- \(\Delta x_{i}\) :
-
Deviation between the experimental and calculated property x of component i
- η :
-
Turbine (or pump) isentropic efficiency
- η ORC :
-
Thermal efficiency of the ORC
References
Mokhtari, H., Sepahvand, M., Fasihfar, A.: Thermoeconomic and exergy analysis in using hybrid systems (GT+MED+RO) for desalination of brackish water in Persian Gulf. Desalination 399, 1–15 (2016). https://doi.org/10.1016/j.desal.2016.07.044
Chadegani, E.A., Sharifishourabi, M., Hajiarab, F.: Comprehensive assessment of a multi-generation system integrated with a desalination system: modeling and analyzing. Energy Convers. Manage. 174, 2C0-32 (2018). https://doi.org/10.1016/j.enconman.2018.08.011
Kasaeian, A., Bellos, E., Shamaeizadeh, A., Tzivanidis, C.: Solar-driven polygeneration systems: recent progress and outlook. Appl. Energy 264, 114764 (2020). https://doi.org/10.1016/j.apenergy.2020.114764
Palenzuela, P., Alarcón-Padilla, D.C., Zaragoza, G.: Large-scale solar desalination by combination with CSP: techno-economic analysis of different options for the Mediterranean Sea and the Arabian Gulf. Desalination 366, 130–138 (2015). https://doi.org/10.1016/j.desal.2014.12.037
Palenzuela, P., Ortega-Delgado, B., Alarcón-Padilla, D.C.: Comparative assessment of the annual electricity and water production by concentrating solar power and desalination plants: a case study. Appl. Therm. Eng. 177, 115485 (2020). https://doi.org/10.1016/j.applthermaleng.2020.115485
Zheng, Y., Caceres Gonzalez, R., Hatzell, M.C., Hatzell, K.B.: Concentrating solar thermal desalination: performance limitation analysis and possible pathways for improvement. Appl. Therm. Eng. 184, 116292 (2021). https://doi.org/10.1016/j.applthermaleng.2020.116292
Jaubert, H., Borel, P., Guichardon, P., Portha, J.F., Jaubert, J.N., Coniglio, L.: Assessment of organic Rankine cycle configurations for solar polygeneration orientated to electricity production and desalination. Appl. Therm. Eng. 195, 116983 (2021). https://doi.org/10.1016/j.applthermaleng.2021.116983
Raman, J.K., Alves, C.M., Gnansounou, E.: A review on moringa tree and vetiver grass—Potential biorefinery feedstocks. Bioresour. Technol. 249, 1044–1051 (2018). https://doi.org/10.1016/j.biortech.2017.10.094
Kumar, B., Verma, P.: Biomass-based biorefineries: an important architype towards a circular economy. Fuel 288, 119622 (2021). https://doi.org/10.1016/j.fuel.2020.119622
Chen, J., Bian, X., Rapp, G., Lang, J., Montoya, A., Trethowan, R., Bouyssiere, B., Portha, J.F., Jaubert, J.N., Pratt, P., Coniglio, L.: From ethyl biodiesel to biolubricants: options for an Indian mustard integrated biorefinery toward a green and circular economy. Ind. Crops Prod. 137, 597–614 (2019). https://doi.org/10.1016/j.indcrop.2019.04.041
Albuquerque, A.A., Ng, F.T.T., Danielski, L., Stragevitch, L.: Phase equilibrium modeling in biodiesel production by reactive distillation. Fuel 271, 117688 (2020). https://doi.org/10.1016/j.fuel.2020.117688
Rapp, G., Garcia-Montoto, V., Bouyssiere, B., Thiebaud Roux, S., Montoya, A., Trethowan, R., Pratt, P., Mozet, K., Coniglio, L.: Dry-purification by natural adsorbents of indian mustard seed oil ethyl biodiesel and biolubricants: toward a low-cost and environmentally-friendly production route. European Biomass Conference and Exhibition Proceedings, pp 621–624 (2020)
Rapp, G., Garcia-Montoto, V., Bouyssiere, B., Thiebaud-Roux, S., Montoya, A., Trethowan, R., Pratt, P., Mozet, K., Portha, J.F., Coniglio, L.: Indian mustard bioproducts dry-purification with natural adsorbents—A biorefinery for a green circular economy. J. Cleaner Prod. 286, 125411 (2021). https://doi.org/10.1016/j.jclepro.2020.125411
Bruno, J.C., Lopez-Villada, J., Letelier, E., Romera, S., Coronas, A.: Modelling and optimisation of solar organic rankine cycle engines for reverse osmosis desalination. Appl. Therm. Eng. 28, 2212–2226 (2008). https://doi.org/10.1016/j.applthermaleng.2007.12.022
Bowskill, D.H., Tropp, U.E., Gopinath, S., Jackson, G., Galindo, A., Adjiman, C.S.: Beyond a heuristic analysis: integration of process and working-fluid design for organic Rankine cycles. Mol. Syst. Des. Eng. 5, 493 (2020). https://doi.org/10.1039/C9ME00089E
Coniglio, L., Coutinho, J.A.P., Clavier, J.Y., Jolibert, F., Jose, J., Mokbel, I., Pillot, D., Pons, M.N., Sergent, M., Tschamber, V.: Biodiesel via supercritical ethanolysis within a global analysis “Feedstocks-conversion-engine” for a sustainable fuel alternative. Prog. Energy Combust. Sci. 43, 1–35 (2014). https://doi.org/10.1016/j.pecs.2014.03.001
Muhammad, F., Oliveira, M.B., Pignat, P., Jaubert, J.N., Pinho, S.P., Coniglio, L.: Phase equilibrium data and modeling of ethylic biodiesel, with application to a non-edible vegetable oil. Fuel 203, 633–641 (2017). https://doi.org/10.1016/j.fuel.2017.05.007
Roze, F., Pignat, P., Ferreira, O., Pinho, S.P., Jaubert, J.N., Coniglio, L.: Phase equilibria of mixtures involving fatty acid ethyl esters and fat alcohols between 4 and 27 kPa for bioproduct production. Fuel 306, 121304 (2021). https://doi.org/10.1016/j.fuel.2021.121304
Weidlich, U., Gmehling, J.: A modified UNIFAC model. 1. Prediction of VLE, hE, and γ∞. Ind. Eng. Chem. Res. 26, 1372–1381 (1987)
Gmehling, J., Li, J., Schiller, M.: A modified UNIFAC model. 2. Present parameter matrix and results for different thermodynamic properties. Ind. Eng. Chem. Res. 32, 178–193 (1993)
Gmehling, J., Wittig, R., Lohmann, J., Joh, R.: A modified UNIFAC (Dortmund) model. 4. Revision and extension. Ind. Eng. Chem. Res. 41, 1678–1688 (2002)
Nitièma-Yefanova, S., Coniglio, L., Schneider, R., Nébié, R.H.C., Bonzi-Coulibaly, Y.L.: Ethyl biodiesel production from non-edible oils of Balanites aegyptiaca, Azadirachta indica, and Jatropha curcas seeds—Laboratory scale development. Renew. Energy 96, 881–890 (2016). https://doi.org/10.1016/j.renene.2016.04.100
Nitièma-Yefanova, S., Tschamber, V., Richard, R., Thiebaud-Roux, S., Bouyssiere, B., Bonzi-Coulibaly, Y.L., Nébié, R.H.C., Coniglio, L.: Ethyl biodiesels derived from non-edible oils within the biorefinery concept—Pilot scale production & engine emissions. Renew. Energy 109, 634–645 (2017). https://doi.org/10.1016/j.renene.2017.03.058
Alsaleh, M., Abdul-Rahim, A.S., Mohd-Shahwahid, H.O.: Determinants of technical efficiency in the bioenergy industry in the EU28 region. Renew. Sust. Energ. Rev. 78, 1331–1349 (2017). https://doi.org/10.1016/j.rser.2017.04.049
Alsaleh, M., Abdul-Rahim, A.S.: Determinants of cost efficiency of bioenergy industry: evidence from EU28 countries. Renew. Energy 127, 746–762 (2018). https://doi.org/10.1016/j.renene.2018.04.085
Abdulwakil, M.M., Abdul-Rahim, A.S., Alsaleh, M.: Bioenergy efficiency change and its determinants in EU-28 region: evidence using least square dummy variable corrected estimation. Biomass Bioenergy 137, 105569 (2020). https://doi.org/10.1016/j.biombioe.2020.105569
Alsaleh, M., Abdul-Rahim, A.S.: The pathway toward bioenergy growth: does information and communication technology development make a difference in EU economies? Biomass Convers. Biorefin. (2021). https://doi.org/10.1007/s13399-021-01933-9
ProII SimSci, 2018 Schneider Electric Software, version 10.1.2.
Talebbeydokhti, P., Cinocca, A., Cipollone, R., Morico, B.: Analysis and optimization of LT-MED system powered by an innovative CSP plant. Desalination 413, 223–233 (2017). https://doi.org/10.1016/j.desal.2017.03.019
Mata-Torres, C., Escobar, R.A., Cardemil, J.M., Simsek, Y., Matute, J.A.: Solar polygeneration for electricity production and desalination: case studies in Venezuela and northern Chile. Renew. Energy 101, 387–398 (2017). https://doi.org/10.1016/j.renene.2016.08.068
Peng, D.Y., Robinson, D.B.: A new two-constant equation of state. Ind. Eng. Chem. Fundam. 15, 59–64 (1976). https://doi.org/10.1021/i160057a011
Le Guennec, Y., Romain Privat, R., Jaubert, J.N.: Development of the translated-consistent tc-PR and tc-RK cubic equations of state for a safe and accurate prediction of volumetric, energetic and saturation properties of pure compounds in the sub and super-critical domains. Fluid Phase Equilib. 429, 301–312 (2016). https://doi.org/10.1016/j.fluid.2016.09.003
Matsuda, H., Yamada, H., Takahashi, R., Koda, A., Kurihara, K., Tochigi, K., Ochi, K.: Ebulliometric determination and prediction of vapor–liquid equilibria for binary mixtures of ethanol and ethyl hexanoate. J. Chem. Eng. Data 56, 5045–5051 (2011). https://doi.org/10.1021/je200868y
Kontogeorgis, G.M., Voutsas, E., Yakoumis, I., Tassios, D.P.: An equation of state for associating fluids. Ind. Eng. Chem. Res. 35, 4310–4318 (1996). https://doi.org/10.1021/ie9600203
Oliveira, M.B., Follegatti-Romero, L.A., Lanza, M., Batista, F.R.M., Batista, E.A.C., Meirelles, A.J.A.: Low pressure vapor–liquid equilibria modeling of biodiesel related systems with Cubic-Plus-Association (CPA) equation of state. Fuel 133, 224–231 (2014). https://doi.org/10.1016/j.fuel.2014.05.016
Larsen, B.L., Rasmussen, P., Fredenslund, A.: A modified UNIFAC group contribution model for prediction of phase equilibria and heats of mixing. Ind. Eng. Chem. Res. 26, 2274–2286 (1987)
Lucia, U., Grisolia, G.: Exergy inefficiency: an indicator for sustainable development analysis. Energy Rep. 5, 62–69 (2019). https://doi.org/10.1016/j.egyr.2018.12.001
Lucia, U., Grisolia, G.: Cyanobacteria and microalgae: thermoeconomic considerations in biofuel production. Energies 11, 156 (2018). https://doi.org/10.3390/en11010156
Lucia, U., Grisolia, G.: Biofuels from micro-organisms: thermodynamic considerations on the role of electrochemical potential on micro-organisms growth. Appl. Sci. 11, 2591 (2021). https://doi.org/10.3390/app11062591
Grisolia, G., Fino, D., Lucia, U.: Biomethanisation of rice straw: a sustainable perspective for the valorisation of a field residue in the energy sector. Sustainability 14, 5679 (2022). https://doi.org/10.3390/su14095679
Fan, L., Zhang, H., Li, J., Wang, Y., Leng, L., Li, J., Yao, Y., Lu, Q., Yuan, W., Zhou, W.: Algal biorefinery to value-added products by using combined processes based on thermochemical conversion: a review. Algal Res. 47, 101819 (2020). https://doi.org/10.1016/j.algal.2020.101819
Zhang, J., Huo, X., Li, Y., Strathmann, T.J.: Catalytic hydrothermal decarboxylation and cracking of fatty acids and lipids over Ru/C. ACS Sustainable Chem. Eng. 7, 14400–14410 (2019). https://doi.org/10.1021/acssuschemeng.9b00215
Gautam, R., Vinu, R.: Reaction engineering and kinetics of algae conversion to biofuels and chemicals via pyrolysis and hydrothermal liquefaction. React. Chem. Eng. 5, 1320–1373 (2020). https://doi.org/10.1039/d0re00084a
Voutsas, E., Louli, V., Boukouvalas, C., Magoulas, K., Tassios, D.: Thermodynamic property calculations with the universal mixing rule for EoS/GE models: results with the Peng-Robinson EoS and a UNIFAC model. Fluid Phase Equilib. 241, 216–228 (2006). https://doi.org/10.1016/j.fluid.2005.12.028
Zabaloy, M.S., Mabe, G.D.B., Bottini, S.B., Brignole, E.A.: Vapor-liquid equilibria in ternary mixtures of water–alcoohol–non polar gases. Fluid Phase Equilib. 83, 15–166 (1993). https://doi.org/10.1016/0378-3812(93)87018-V
González Prieto, M., Sánchez, F.A., Pereda, S.: Thermodynamic model for biomass processing in pressure intensified technologies. J. Supercritical Fluids 96, 53–67 (2015). https://doi.org/10.1016/j.supflu.2014.08.024
Acknowledgements
The author would like to express his deepest thanks to the following researchers for their fruitful collaborations that led to papers on which this work is partly built: Olga Ferreira, Simão P. Pinho (Mountain Research Center - CIMO, Polytechnic Institute of Bragança, 5301-855 Bragança, Portugal), Graeme Rapp, Richard Trethowan (The University of Sydney, Plant Breeding Institute, I.A. Watson International Grains Research Centre, PO Box 219, Narrabri, NSW 2390, Australia), Hadrien Jaubert, Pierrette Guichardon (Aix Marseille Université, CNRS, Centrale Marseille, M2P2 UMR 7340, Pôle de l’Etoile, Technopôle de Château-Gombert, 38 rue Frédéric Joliot-Curie, 13451 Marseille, France), Patrice Pignat (PIGNAT SAS, 6, rue Calmette, 67740 Genas, France), Frédéric Roze, Jean-Noël Jaubert and Jean-François Portha (Université de Lorraine - ENSIC, Laboratoire Réactions et Génie des Procédés (UMR CNRS 7274), 1 rue Grandville, 54000 Nancy, France)
Author information
Authors and Affiliations
Contributions
Writing - review & editing
Corresponding author
Ethics declarations
Competing Interests
The author declares that she has no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. Also, no funds, grants, or other support was received.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Coniglio, L. Illustrations of the Synergy Between Thermodynamics and Chemical Reaction into the Triptych “Bioproducts-Bioenergy-Water”. J Solution Chem 53, 571–593 (2024). https://doi.org/10.1007/s10953-023-01305-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-023-01305-z