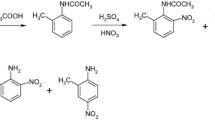
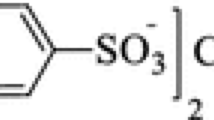Abstract
The method of isothermal saturation was utilized to experimentally obtain the sodium 4-tolylsulfinate (4-STS) solubility in nine neat solvents including ethanol, acetone, methanol, N,N-dimethylformamide (DMF), isopropanol, acetonitrile, water, n-butanol, and n-propanol, and solvent mixtures of water + isopropanol/acetonitrile. With a rise in temperature, the mole fractions of 4-STS in the equilibrium liquor increased. Among the solvents tested, methanol has the largest solubility values. To match the solubility data, the Apelblat, the Jouyban–Acree, and the modified van't Hoff–Jouyban–Acree models were utilized. The values of 9.23 × 10–4 and 5.30 × 10–2 were the highest root-mean-square and relative mean error values, respectively. The recommended anti-solvents for crystallization of the 4-STS from aqueous solution are isopropanol and acetonitrile. The mutual solubility of 4-STS and Na2SO4/NaCl in water was determined at the temperatures from T = 283.15 K to 313.15 K at intervals of 15 K. The phase diagrams of 4-STS + Na2SO4/NaCl + water ternary systems were also constructed. We found that, the addition of a certain amount of Na2SO4/NaCl can significantly decrease the aqueous solubility of 4-STS.





Similar content being viewed by others
References
Prausnitz, J.M., Tavares, F.W.: Thermodynamics of fluid-phase equilibria for standard chemical engineering operations. AIChE J. 50, 739–761 (2004)
Jouyban, A.: Handbook of Solubility Data for Pharmaceuticals. CRC Press, Boca Raton, FL (2010)
Kolar, P., Shen, J.W., Tsuboi, A., Ishikawa, T.: Solvent selection for pharmaceuticals. Fluid Phase Equilib. 194–197, 771–782 (2002)
Hahnenkamp, I., Graubner, G., Gmehling, J.: Measurement and prediction of solubilities of active pharmaceutical ingredients. Int. J. Pharm. 388, 73–81 (2010)
Wu, G.Z., Schumacher, D.P., Tormos, W., Clark, J.E., Murphy, B.L.: An improved industrial synthesis of florfenicol plus an enantioselective total synthesis of thiamphenicol and florfenicol. J. Org. Chem. 62, 2996–2998 (1997)
Joze, G., Ivanka, K.: Process for the synthesis of intermediates of chloramphenicol or its analogues. EP Patent 1,785,414, May 16 (2007)
Wang, H.L., Zhao, L.F., Shen, G.Q., Hu, W.J., Jiang, Q.M., Feng, M.H.: Industrialized production method for methyl p-tolyl sulfone. CN Patent 102,363,604, February 29 (2012)
He, C.H.: Production process of methyl p-tolyl sulfone. CN Patent 101,434,566, May 25 (2009)
Yang, H.Z., Wu, Z.G., Cui, M.L., Yuan, X.B.: Preparation method of para methyl p-tolyl sulfone. CN Patent 101,648,895, February 17 (2010)
Hu, X.H.: Preparing of para methyl p-tolyl sulfone. Zhejiang Chem. Ind. 26, 27–28 (1995). (Chinese)
Cheng, C., Cong, Y., Du, C.B., Wang, J., Yao, G.B., Zhao, H.K.: Solubility determination and thermodynamic models for dehydroepiandrosterone acetate in mixed solvents of (ethyl acetate + methanol), (ethyl acetate + ethanol) and (ethyl acetate + isopropanol). J. Chem. Thermodyn. 101, 372–379 (2016)
Zhu, Y.Q., Ding, G.X., Li, X.B., Zhao, H.K.: Solid–liquid phase equilibrium for ternary systems of p-nitroacetophenone plus m-nitroacetophenone plus methanol/toluene/ethyl acetate. J. Chem. Eng. Data 64, 4066–4076 (2019)
Yao, G.B., Yao, Q.C., Xia, Z.X., Li, Z.H.: Solubility determination and correlation for o-phenylenediamine in (methanol, ethanol, acetonitrile and water) and their binary solvents from T = (283.15–318.15) K. J. Chem. Thermodyn. 105, 179–186 (2017)
Han, S., Meng, L., Du, C.B., Xu, J., Cheng, C., Wang, J., Zhao, H.K.: Solubility measurement and thermodynamic modeling of 4-nitrophthalimide in twelve pure solvents at elevated temperatures ranging from (273.15 to 323.15) K. J. Chem. Eng. Data 61, 2525–2535 (2016)
Schott, H.: A Mathematical extrapolation for the method of wet residues. J. Chem. Eng. Data 6, 324–324 (1961)
Yao, G.B., Xia, Z.X., Li, Z.H., Shao, C.: Determination and modeling for solid-liquid phase equilibrium of ternary caprolactam + cyclohexanone oxime + methyl tert-butyl ether system. Fluid Phase Equilib. 417, 242–247 (2016)
Apelblat, A., Manzurola, E.: Solubilities of o-acetylsalicylic, 4-aminosalicylic, 3,5-dinitrosalicylic, and p-toluic acid, and magnesium-dl-aspartate in water from T = (278 to 348) K. J. Chem. Thermodyn. 31, 85–91 (1999)
Yao, G.B., Xia, Z.X., Li, Z.H.: Thermodynamic study of solubility for 2-amino-4-chloro-6-methoxypyrimidine in twelve organic solvents at temperatures from 273.15 K to 323.15 K. J. Chem. Thermodyn. 105, 187–197 (2017)
Yao, G.B., Yao, Q.C., Xia, Zh.X., Li, Zh.H.: Solubility determination and thermodynamic modelling of 3,5-dimethylpyrazole in nine organic solvents from T = (283.15 to 313.15) K and mixing properties of solutions. J. Chem. Thermodyn. 110, 99–109 (2017)
Yuan, Y., Zheng, M., Zhao, H.K., Kong, L.M.: Solubility determination and modeling of p-nitrobenzamide dissolved in twelve neat solvents from 283.15 to 328.15 K. J. Chem. Eng. Data 64, 1840–1850 (2019)
Wang, H.J., Yao, G.B., Zhang, H.J.: Measurement and correlation of the solubility of baicalin in several mixed solvents. J. Chem. Eng. Data 64, 1281–1287 (2019)
Jouyban, A.: Review of the cosolvency models for predicting drug solubility in solvent mixtures: an update. J. Pharm. Pharm. Sci. 22, 466–485 (2019)
Yao, G.B., Li, Z.H., Xia, Z.X., Yao, Q.C.: Solubility of N-phenylanthranilic acid in nine organic solvents from T = (283.15 to 318.15) K: determination and modeling. J. Chem. Thermodyn. 103, 218–227 (2016)
Martinez, F., Acree, W.E., Jr., Jouyban, A.: Correct derivation of a combined version of the Jouyban-Acree and van’t Hoff model and some comments on ’Determination and correlation of the solubility of myricetin in ethanol and water mixtures from 288.15 to 323.15 K. Phys. Chem. Liq. 55, 131–140 (2017)
Lu, J., Zhou, X., Chen, L.W., Zhang, L.J., Rohani, S.: Solid−liquid equilibria of the Na2SO4 + H2NCH2CH2SO3H + H2O system from (288.15 to 328.15) K. J. Chem. Eng. Data 59, 2115–2119 (2014)
Cadbury, W.E., Jr.: The system sodium sulfate–sodium molybdate–water. J. Phys. Chem. 59, 257–260 (1955)
Chen, S.Q., Cui, W.J., Hu, J.Y., Guo, Y.F., Deng, T.L.: Phase equilibria and phase diagrams for the aqueous ternary system containing sodium, sulfate, and metaborate ions at 288.15 and 308.15 K and 101.325 kPa. J. Chem. Eng. Data 64, 2809–2815 (2019)
Li, D.C., Fan, R., Guo, X.F., Yang, S.N., Zhang, Z.Y.: Phase equilibria in the aqueous ternary system (NH4)2SO4 + Na2SO4 + H2O at T = (303.15 and 313.15) K and p = 0.1 MPa. J. Chem. Eng. Data 63, 635–641 (2018)
Chen, X.Z., Tang, J.H., Cui, Y.N., Liu, M., Mao, D.: Phase equilibrium for the ternary system NaH2PO4 + Na2SO4 + H2O in aqueous solution at 298.15 K. J. Chem. Eng. Data 59, 481–484 (2014)
Pinho, S.P., Macedo, E.A.: Solubility of NaCl, NaBr, and KCl in water, methanol, ethanol, and their mixed solvents. J. Chem. Eng. Data 50, 29–32 (2005)
Farelo, F., von Brachel, G., Offermann, H.: Solid-liquid equilibria in the ternary system NaCl-KCl–H2O. Can. J. Chem. Eng. 71, 141–146 (1993)
Zeng, Y., Li, Z.B.: Solubility measurement and modeling for the NaCl−NH4Cl−monoethylene glycol−H2O system from (278 to 353) K. J. Chem. Eng. Data 60, 2248–2255 (2015)
Zhang, X.R., Ren, Y.S., Li, P., Ma, H.J., Ma, W.J., Liu, C.Q., Wang, Y.N., Kong, L.X., Shen, W.: Solid−liquid equilibrium for the ternary systems (Na2SO4 + NaH2PO4 + H2O) and (Na2SO4 + NaCl + H2O) at 313.15 K and atmospheric pressure. J. Chem. Eng. Data 59, 3969–3974 (2014)
Wang, Q.X., Li, Z.B.: Solubility determination and thermodynamic modeling for the system NaCl–NH4Cl–Diethanolamine–H2O. J. Chem. Eng. Data 64, 895–904 (2019)
Héctor, R.G., María, E.T., Teófilo, A.G.: Compositions, densities, and refractive indices of potassium chloride + ethanol + water and sodium chloride + ethanol + water solutions at (298.15 and 313.15) K. J. Chem. Eng. Data 48, 405–410 (2003)
Li, R.R., Zhao, H.K., Jiang, S.N., Sun, L.L.: Solubility of 1,6-naphthalene disulfonic acid disodium in binary sodium chloride + water, sodium sulfate + water, and ethanol + water solvent mixtures at elevated temperatures. J. Chem. Eng. Data 56, 2692–2695 (2011)
Acknowledgements
We sincerely express our thanks to the Doctoral Research Start-up Fund project (NO. NGBJ-2020-32) of Nanyang Institute of Technology and Nanyang City Science and technology project of 2020 year (JCQY009).
Author information
Authors and Affiliations
Contributions
HL wrote the main manuscript text and HZ, YL, and HZ prepared figures 1-5. Other authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
We declare that we do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, H., Zhang, H., Li, Y. et al. Investigation of the Solubility and Phase Diagrams of Sodium 4-Tolylsulfinate in Different Solvents and Ternary Sodium 4-Tolylsulfinate + Sodium Chloride/Sodium Sulfate + Water Systems. J Solution Chem 52, 790–804 (2023). https://doi.org/10.1007/s10953-023-01272-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-023-01272-5