Abstract
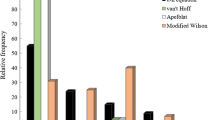
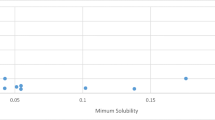
The current study validated some trained models for predicting clozapine solubility data in mono- and binary-solvent mixtures. The prediction models were built by combining the van't Hoff and Jouyban–Acree models with various physicochemical characteristics such as Abraham solvation parameters and Catalan and Hansen solubility parameters. The overall mean relative deviation and cross-validation analyses were used to assess the models' accuracy and applicability. Validated models can be used to estimate the clozapine solubility in mono-solvents or solvent mixtures at various temperatures.



Similar content being viewed by others
References
Hippius, H.: The history of clozapine. Psychopharmacology 15, 25–50 (1989)
Huhn, M., Nikolakopoulou, A., Schneider-Thoma, J., Krause, M., Samara, M., Peter, N., Arndt, T., Bäckers, L., Rothe, P., Cipriani, A.: Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: a systematic review and network meta-analysis. Lancet 394, 939–951 (2019)
Wagstaff, A.J., Perry, C.M.: Clozapine. CNS Drugs 17, 273–280 (2003)
Taipale, H., Tanskanen, A., Mehtälä, J., Vattulainen, P., Correll, C.U., Tiihonen, J.: 20-year follow-up study of physical morbidity and mortality in relationship to antipsychotic treatment in a nationwide cohort of 62,250 patients with schizophrenia (FIN20). World Psychiatry 19, 61–68 (2020)
Singh, R.: Global Clozapine Market Professional Survey Report. 2019.
Dave, R.A., Morris, M.E.: Novel high/low solubility classification methods for new molecular entities. Int. J. Pharm. 511, 111–126 (2016)
Benet, L.Z., Broccatelli, F., Oprea, T.I.: BDDCS applied to over 900 drugs. AAPS J. 13, 519–547 (2011)
Gerald, K.M.: AHFS drug information, pp. 2273–2285. American Society of Health-System Pharmacists Press, Bethesda (2004)
Gong, Y., Wang, Y., Huang, Z., Li, Y., Li, T., Ren, B.: Solubility, solvent effect, molecular simulation and thermodynamic properties of clozapine in twelve pure solvents. J. Chem. Thermodyn. 158, 106398 (2021)
Huang, W., Wang, H., Li, C., Wen, T., Xu, J., Ouyang, J., Zhang, C.: Measurement and correlation of solubility, Hansen solubility parameters and thermodynamic behavior of clozapine in eleven mono-solvents. J. Mol. Liq. 333, 115894 (2021)
Yu, S., Cheng, Y., Du, S., Wang, Y., Xue, F., Xing, W.: Thermodynamic analysis of the solubility of clozapine in organic solvents. J. Chem. Thermodyn. 158, 106451 (2021)
Li, C., Wang, H., Huang, W., Wen, T., Xu, J., Ouyang, J., Zhang, C.: Solubility measurement, modeling and Hansen solubility parameters of 8-Chloro-11-(4-methyl-1-piperazinyl)-5H-dibenzo [b, e][1, 4] diazepine in four binary solvents. J. Mol. Liq. 339, 116733 (2021)
Yu, S., Yuan, J., Cheng, Y., Du, S., Wang, Y., Xue, F., Xing, W.: Solid-liquid phase equilibrium of clozapine in aqueous binary solvent mixtures. J. Mol. Liq. 329, 115371 (2021)
Yao, X.D., Wang, Z., Geng, Y., Zhao, H.K., Rahimpour, E., Acree, W.E., Jr., Jouyban, A.: Hirshfeld surface and electrostatic potential surface analysis of clozapine and its solubility and molecular interactions in aqueous blends. J. Mol. Liq. 360, 119328 (2022)
Jouyban, A., Fakhree, M.A.A., Shayanfar, A.: Solubility prediction methods for drug/drug like molecules. Recent Innov. Chem. Eng. 1, 220–231 (2008)
Jouyban-Gharamaleki, V., Jouyban, A., Acree, W.E., Jr., Rahimpour, E.: Smart systems for determination of drug’s solubility. Drug Dev. Ind. Pharm. 45, 177–187 (2019)
Jouyban-Gharamaleki, A., Valaee, L., Barzegar-Jalali, M., Clark, B., Acree, W.E., Jr.: Comparison of various cosolvency models for calculating solute solubility in water–cosolvent mixtures. Int. J. Pharm. 177, 93–101 (1999)
Adjei, A., Newburger, J., Martin, A.: Extended Hildebrand approach: solubility of caffeine in dioxane–water mixtures. J. Pharm. Sci. 69, 659–661 (1980)
Yalkowsky, S.H., Roseman, T.J.: Solubilization of drugs by cosolvents. In: Techniques of Solubilization of Drugs, vol. 12, pp. 91–134. M. Dekker, New York (1981)
Williams, N., Amidon, G.: Excess free energy approach to the estimation of solubility in mixed solvent systems III: ethanol–propylene glycol–water mixtures. J. Pharm. Sci. 73, 18–23 (1984)
Ochsner, A.B., Belloto, R.J., Jr., Sokoloski, T.D.: Prediction of xanthine solubilities using statistical techniques. J. Pharm. Sci. 74, 132–135 (1985)
Acree, W.E., Jr.: Mathematical representation of thermodynamic properties: part 2. Derivation of the combined nearly ideal binary solvent (NIBS)/Redlich–Kister mathematical representation from a two-body and three-body interactional mixing model. Thermochim. Acta. 198, 71–79 (1992)
Khossravi, D., Connors, K.A.: Solvent effects on chemical processes, I: solubility of aromatic and heterocyclic compounds in binary aqueous—organic solvents. J. Pharm. Sci. 81, 371–379 (1992)
Jouyban-Gharamaleki, A.: The modified Wilson model and predicting drug solubility in water-cosolvent mixtures. Chem. Pharm. Bull. 46, 1058–1061 (1998)
Jouyban, A., Acree, W.E., Jr.: Mathematical derivation of the Jouyban–Acree model to represent solute solubility data in mixed solvents at various temperatures. J. Mol. Liq. 256, 541–547 (2018)
Ruckenstein, E., Shulgin, I.: Solubility of drugs in aqueous solutions: Part 2: Binary nonideal mixed solvent. Int. J. Pharm. 260, 283–291 (2003)
Ruidiaz, M.M.A., Rodríguez, D.S.J., Neita, R.P.C., Cristancho, B.D.M., Martínez, R.F.: Performance of the Jouyban–Acree model for correlating the solubility of indomethacin and ethylhexyl triazone in ethyl acetate + ethanol mixtures. Vitae 17, 309–316 (2010)
Zhao, X., Farajtabar, A., Zhao, H.K., Han, G.: Solubility and solvent effect of acetamiprid in thirteen pure solvents and aqueous solutions of ethanol. J. Chem. Eng. Data 64, 3505–3513 (2019)
Li, X.B., He, Y.T., Xu, Y.Y., Zhang, X.T., Zheng, M., Zhao, H.K.: 5-Nitrosalicylaldehyde in aqueous co-solvent mixtures of methanol, ethanol, isopropanol and acetonitrile: solubility determination, solvent effect and preferential solvation analysis. J. Chem. Thermodyn. 142, 106014 (2020)
Li, W.X., Farajtabar, A., Xing, R., Zhu, Y.T., Zhao, H.K.: Equilibrium solubility determination, solvent effect and preferential solvation of amoxicillin in aqueous co-solvent mixtures of N,N-dimethylformamide, isopropanol, N-methyl pyrrolidone and ethylene glycol. J. Chem. Thermodyn. 142, 106010 (2020)
Li, W.X., Farajtabar, A., Xing, R., Zhu, Y.T., Zhao, H.K., Lv, R.G.: 3-Methyl-6-nitroindazole in some aqueous co-solvent mixtures: solubility determination, preferential solvation and solvent effect analysis. J. Chem. Thermodyn. 144, 106066 (2020)
Cong, Y., Du, C.B., Wang, J., Zhao, H.K.: Determination and thermodynamic modelling for 2-methyl-6-nitroaniline solubility in binary solvent mixtures of ethyl acetate + (methanol, ethanol, n-propanol and isopropanol). J. Chem. Thermodyn. 105, 404–413 (2017)
Zhang, C.L., Jouyban, A., Zhao, H.K., Farajtabar, A., Acree, W.E., Jr.: Equilibrium solubility, Hansen solubility parameter, dissolution thermodynamics, transfer property and preferential solvation of zonisamide in aqueous binary mixtures of ethanol, acetonitrile, isopropanol and N,N-dimethylformamide. J. Mol. Liq. 326, 115219 (2021)
Yuan, Y., Farajtabar, A., Kong, L.M., Zhao, H.K.: Thermodynamic solubility modelling, solvent effect and preferential solvation of p-nitrobenzamide in aqueous co-solvent mixtures of dimethyl sulfoxide, ethanol, isopropanol and ethylene glycol. J. Chem. Thermodyn. 136, 123–131 (2019)
Li, Y.X., Mou, Y., Zhu, Y., Liu, J.H., Liu, J., Zhao, H.K.: Equilibrium solubility determination and thermodynamic aspects of aprepitant (form I) in four binary aqueous mixtures of methanol, ethanol, acetone and 1,4-dioxane. J. Chem. Thermodyn. 149, 106170 (2020)
Jouyban, A., Soltanpour, S., Soltani, S., Chan, H., Acree, W.E., Jr.: Solubility prediction of drugs in water-cosolvent mixtures using Abraham solvation parameters. J. Pharm. Pharm. Sci. 10, 263–277 (2007)
Jouyban, A., Soltanpour, S., Soltani, S., Tamizi, E., Fakhree, M.A.A., Acree, W.E., Jr.: Prediction of drug solubility in mixed solvents using computed Abraham parameters. J. Mol. Liq. 146, 82–88 (2009)
Jouyban, A., Shayanfar, A., Ghafourian, T., Acree, W.E., Jr.: Solubility prediction of pharmaceuticals in dioxane+ water mixtures at various temperatures: effects of different descriptors and feature selection methods. J. Mol. Liq. 195, 125–131 (2014)
Rahimpour, E., Barzegar-Jalali, M., Shayanfar, A., Jouyban, A.: Generally trained models to predict drug solubility in N-methyl-2-pyrrolidone + water mixtures at various temperatures. J. Mol. Liq. 254, 34–38 (2018)
Barzegar-Jalali, M., Rahimpour, E., Martinez, F., Jouyban, A.: Generally trained models to predict drug solubility in methanol+ water mixtures. J. Mol. Liq. 264, 631–644 (2018)
Mohammadian, E., Barzegar-Jalali, M., Rahimpour, E.: Solubility prediction of lamotrigine in cosolvency systems using Abraham and Hansen solvation parameters. J. Mol. Liq. 276, 675–679 (2019)
Mohammadian, E., Jouyban, A., Barzegar-Jalali, M., Acree, W.E., Jr., Rahimpour, E.: Solubilization of naproxen: experimental data and computational tools. J. Mol. Liq. 288, 110985 (2019)
Rahimpour, E., Mohammadian, E., Acree, W.E., Jr., Jouyban, A.: Computational tools for solubility prediction of celecoxib in the binary solvent systems. J. Mol. Liq. 299, 112129 (2020)
Rahimpour, E., Jouyban, A.: Utilizing Abraham and Hansen solvation parameters for solubility prediction of meloxicam in cosolvency systems. J. Mol. Liq. 328, 115400 (2021)
Aerts, J.: The Hoy solubility parameter calculation software. Computer Chemistry Counsultancy, Singen, Germany, Germany (2005)
Hansen, C., Parameters, H.S.: A user’s handbook. CRC Press, Boca Raton, Fl (2000)
Acree, W.E., Jr., Grubbs, L.M., Abraham, M.H.: Prediction of partition coefficients and permeability of drug molecules in biological systems with Abraham model solute descriptors derived from measured solubilities and water-to-organic solvent partition coefficients, pp. 100–102. InTech. Publisher, New York (2012)
Grant, D., Mehdizadeh, M., Chow, A.-L., Fairbrother, J.: Non-linear van’t Hoff solubility-temperature plots and their pharmaceutical interpretation. Int. J. Pharm. 18, 25–38 (1984)
Jouyban, A., Rahimpour, E., Karimzadeh, Z.: A new correlative model to simulate the solubility of drugs in mono-solvent systems at various temperatures. J. Mol. Liq. 343, 117587 (2021)
Acknowledgements
We are grateful to Tabriz University of Medical Sciences for the financial support of this research (Grant No: 67898).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rahimpour, E., Xu, R., Zhao, H. et al. Simulation of Clozapine Solubility in Mono- and Mixed Solvents at Different Temperatures. J Solution Chem 51, 1540–1570 (2022). https://doi.org/10.1007/s10953-022-01208-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-022-01208-5