Abstract
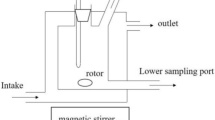
To separate the azeotrope of 2-methyl-2-butanol and water, n-heptanol was selected as the extractant. Liquid–liquid equilibrium (LLE) data for the ternary system of 2-methyl-2-butanol + water + n-heptanol were measured at 303.15 K, 313.15 K and 323.15 K under 101.3 kPa. The quality of the experimental LLE data was verified by the Hand equation, the Othmer–Tobias equation, and the mass balance between the feed and the conjugated phases. The results show that the correlation coefficients (R2) of the equations are close to 1, the relative error of mass balance is less than 0.7%. The selectivity (S) was used to represent the ability of n-heptanol to extract 2-methyl-2-butanol from water, which is much greater than 133. The experimental data show that S decreases with the mass fraction of 2-methyl-2-butanol in aqueous phase, and increases with equilibrium temperature. The NRTL and UNIQUAC models were used to regress the experimental LLE data, and the corresponding binary interaction parameters were obtained. The root-mean square deviations (RMSDs) indicate that the UNIQUAC model performs better than the NRTL model.






Similar content being viewed by others
References
Zhou, Y., Chen, H.J., Zhang, R., Ding, Z.X., Tang, Q.Y., Yang, Z.Y., Sun, R.H., Fan, C.L., Sun, C.S., He, K., Bai, M.: Preparation of tert-amyl alcohol from isopentene. CN Patent 111(777), 490A (2020)
Chen, H.J., Hu, G.J., Qi, Z.J., Li, Y., Xu, X.H., Lu, X.G., Zheng, Z.: Method for preparation of tert-amyl alcohol by isoamylene hydration. CN Patent 108(017), 508A (2018)
Yao, Z.L., Zhao, Y.Z., Dun, J.T.: Method for preparation of tert-amyl alcohol. CN Patent 1(374), 284A (2002)
Fischer, K., Shulgin, I., Rarey, J., Gmehling, J.: Vapor–liquid equilibria for the system water + tert-pentanol at 4 temperatures. Fluid Phase Equilib. 120, 143–165 (1996)
El-Dossoki, F.I.: The influence of cation, anion and temperature on the liquid–liquid equilibrium of some pentanols–water system. Fluid Phase Equilib. 305, 161–168 (2011)
Gomis, V., Ruiz, F., Boluda, N., Saquete, M.D.: Liquid–liquid-solid equilibria for ternary systems water + sodium chloride + pentanols. J. Chem. Eng. Data 44, 918–920 (1999)
Boluda, N., Gomis, V., Ruiz, F., Saquete, M.D., Barnes, N.: Liquid–liquid-solid equilibria for ternary systems of water + potassium chloride + pentanols. Fluid Phase Equilib. 179, 269–276 (2001)
Gomis, V., Ruiz, F., Boluda, N., Saquete, M.D.: Liquid–liquid–solid equilibria for ternary systems water + lithium chloride + pentanols. Fluid Phase Equilib. 215, 79–83 (2004)
Jiang, M., Chen, Y., Shen, S.: Liquid–liquid equilibria for octan-2-one + dihydroxybenzene + water at different temperatures: experimental data and thermodynamic modeling. J. Chem. Eng. Data 64, 4454–4464 (2019)
Shen, S., Chen, Y., Jiang, M.: Experiments and COSMO-SAC modeling of methyl isobutyl ketone + dimethylphenols + water mixtures. J. Chem. Eng. Data 64, 3521–3534 (2019)
Xu, D., Zhang, L., Gao, J., Pratik, D., Zhao, L., Cui, Z.: Liquid–liquid equilibrium for ternary systems of ethyl acetate / isopropyl acetate + 2,2,3,3-tetrafluoro-1-propanol + water at 298.15, 318.15 K. J. Chem. Thermodyn. 106, 218–227 (2017)
Li, A., Xu, X., Zhang, L., Gao, J., Xu, D., Wang, Y.: Separation of cresol from coal tar by imidazolium-based ionic liquid [Emim] [SCN]: Interaction exploration and extraction experiment. Fuel 264, 116908 (2020)
Zhang, X., Wang, Z., Wang, K., Reyes-Labarta, J.A., Gao, J.: Liquid–liquid phase equilibrium and interaction exploration for separation of azeotrope (2,2,3,3-tetrafluoro-1-propanol + water) with two imidazolium-based ionic liquids. J. Mol. Liq. 300, 112266 (2020)
Wang, P., Yan, P., Reyes-Labarta, J.A., Gao, J., Xu, D., Zhang, L., Wang, Y.: Liquid–liquid measurement and correlation for separation of azeotrope (dimethyl carbonate and ethanol) with different imidazolium-based ionic liquids. Fluid Phase Equilib. 485, 183–189 (2019)
Cháfer, A., de la Torre, J., Lladosa, E., Montón, J.B.: Measurements and correlation at different temperatures of liquid–liquid equilibria of 2-butanol or 2-methyl-2-butanol + 1,2,3-propanetriol + water ternary systems. Fluid Phase Equilib. 377, 38–44 (2014)
Dai, F., Zhao, M., Jia, M.: Liquid–liquid equilibrium study for ternary systems of water + propargyl alcohol + solvents at 308.2 K: measurement and thermodynamic modeling. J. Chem. Thermodyn. 135, 149–154 (2019)
Männistö, M., Pokki, J.-P., Creati, A., Voisin, A., Zaitseva, A., Alopaeus, V.: Ternary and binary LLE measurements for solvent (4-methyl-2-pentanone and 2-methyl-2-butanol) + furfural + water between 298 and 401 K. J. Chem. Eng. Data 61, 903–911 (2016)
Chiou, D., Chen, L.: Liquid–liquid equilibria for the ternary system water + diethylene glycol monohexyl ether + 2-methyl-2-butanol. Fluid Phase Equilib. 218, 229–234 (2004)
Chiou, D., Chen, L.: Liquid–liquid equilibria for the ternary system water + 2-methyl-2-butanol + diethyleneglycol monobutylether. J. Chem. Eng. Data 46, 1530–1532 (2001)
Pai, Y., Chen, L.: Liquid–liquid equilibria of two binary systems: water + 1-pentanol and water + 2-methyl-2-butanol and two ternary systems: water + 1-pentanol + 2-butyloxyethanol and water + 2-methyl-2-butanol + 2-butyloxyethanol. Fluid Phase Equilib. 155, 95–105 (1999)
Zhang, X., Yang, X., Jian, J., Jian, C., He, J., Wang, H., Kong, W., Shangguan, G., Xia, M.: Liquid–liquid equilibrium for 2-methyl-2-butanol + water + o-xylene at different temperatures. J. Chem. Eng. Data 65, 5090–5095 (2020)
Zhang, X., Yang, F., Jian, J., Jian, C., Wang, H., Kong, W., Shangguan, G., Xia, M.: Liquid–liquid equilibrium for the ternary system of (2-methyl-2-butanol + water + n-octanol) at different temperatures. J. Chem. Thermodyn. 161, 106522 (2021)
Zhang, X., Jian, C., Du, J., Wang, H., Wang, H., Kong, W., Shangguan, G., Xia, M., Lu, H.: Measurements and thermodynamic modeling of liquid–liquid equilibrium data for 2-methyl-2-butanol + water + ethylbenzene. J. Chem. Eng. Data 66, 2153–2159 (2021)
ChemBlink: https://www.chemblink.com/MSDS/MSDSFiles/111-70-6_Sigma-Aldrich.pdf.
Dong, Y., Zhu, R., Gao, Y., Lei, Z.: A united chemical thermodynamic model: COSMO-UNIFAC. Ind. Eng. Chem. Res. 57, 15954–15958 (2018)
Zhu, R., Taheri, M., Zhang, J., Lei, Z.: Extension of the COSMO-UNIFAC thermodynamic model. Ind. Eng. Chem. Res. 59, 1693–1701 (2020)
Aspen Plus Software: Version 11. Aspen Technology Inc, Burlington, MA (2019)
Dai, F., Xin, K., Song, Y., Shi, M., Yu, Y., Li, Q.: Liquid–liquid equilibria for the ternary system containing 1-butanol + methoxy (methoxymethoxy) + water at temperatures of 303.15, 323.15 and 343.15 K. Fluid Phase Equilib. 409, 466–471 (2016)
JCGM: GUM 1995 with minor corrections. Evaluation of Measurement Data-Guide to the Expression of Uncertainty in Measurement 100, 2008 (2008)
Marongiu, B., Ferino, I., Monaci, R., Solinas, V., Torrazza, S.: Thermodynamic properties of aqueous non-electrolyte mixtures. alkanols + water systems. J. Mol. Liq. 28, 229–247 (1984)
Ginnings, P.M., Baum, R.: Aqueous solubilities of the isomeric pentanols. J. Am. Chem. Soc. 59, 1111–1113 (1937)
Adel, S.A., Aljimaz, S.H.F., Jasem, A.A., Mohamed, A.F.: Liquid–liquid equilibria of the ternary system water + acetic acid + 1-heptanol. J. Chem. Eng. Data 45, 301–303 (2000)
Darwish, N.A., Abdulkarim, M.A., Ashour, I., Dwaidar, A.M., Athamneh, F.S.: Liquid–liquid equilibrium for the system water + acetic acid + 1-heptanol at 278.1, 293.1, 303.1 and 313.1 K. Fluid Phase Equilib. 200, 277–285 (2002)
Richard, S., James, S., Mary, T.: Mutual solubility of water and aliphatic alcohols. J. Chem. Eng. Data 29, 287–290 (1984)
Goral, M., Wisniewska-Goclowska, B.: IUPAC-NIST solubility data series. 82. alcohols with water. Part 4. C7 alcohols with water. J. Phys. Chem. Ref. Data 36, 445–484 (2007)
Othmer, D.F., Tobias, P.E.: Liquid–liquid extraction data–Toluene and acetaldehyde systems. Ind. Eng. Chem. 34, 693–696 (1942)
Hand, D.B.: The distribution of consolute liquid between two immiscible liquids. J. Phys. Chem. 34, 1961–2000 (1930)
Marcilla, A., Ruiz, F., García, A.N.: Liquid–liquid-solid equilibria of the quaternary system water-ethanol-acetone-sodium chloride at 25 ℃. Fluid Phase Equilib. 112, 273–289 (1995)
ChemBlink: https://www.chemblink.com/MSDS/MSDSFiles/95-47-6_Sigma-Aldrich.pdf.
ChemBlink: https://www.chemblink.com/MSDS/MSDSFiles/100-41-4_Sigma-Aldrich.pdf
Renon, H., Prausnitz, J.M.: Local compositions in thermodynamic excess functions for liquid mixtures. AIChE J. 14, 135–144 (1968)
Abrams, D.S., Prausnitz, J.M.: Statistical thermodynamics of liquid mixtures: a new expression for the excess gibbs energy of partly or completely miscible systems. AIChE J. 21, 116–128 (1975)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, X., Yang, F., Jian, C. et al. Liquid–Liquid Equilibrium Study of the Ternary System 2-Methyl-2-butanol + Water + n-Heptanol at 303.15 K, 313.15 K and 323.15 K. J Solution Chem 51, 224–239 (2022). https://doi.org/10.1007/s10953-022-01144-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-022-01144-4