Abstract
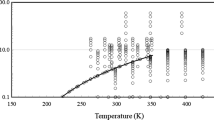
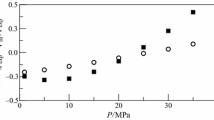
Experimental saturated vapor pressure data were measured using a Modified Swietoslawski-type ebulliometer and correlated with the Antoine, Clarke–Glew, and Wagner equations. A least root-mean-square deviation (RMSD) was obtained for the Antoine (0.667) and Wagner (0.796) equations while the group contribution and group interaction (GCGI) method by Nanoolal et al. showed an RMSD of 1.094. The estimated enthalpy of vaporization is found to be 46.87 kJ·mol−1 and 38.56 kJ·mol−1 at reference temperature (298.15 K) and normal boiling point (422.28 K), respectively, and was well verified with Watson’s correlation. The critical properties and acentric factor are reported based on the GCGI method. Experimental density data are reported and are well correlated with the DIPPR 116 correlation with an RMSD of 0.00416. Joback’s method predicted the densities well with a maximum absolute deviation (MAD) of 0.00548. The Vogel–Tamman–Fulcher equation correlated the experimental viscosities in the temperature range of 288.15–338.15 K with a MAD of 0.002. The isothermal expansivity for tribromomethane shows a linear trend with a weak temperature dependency.










Similar content being viewed by others
References
Harlow, I.F., Ross, O.C.: Preparation of brominated hydrocarbons. US patent 1891415A (1932)
Dauphin, G., Kergomard, A.A.: The acid dissociation of some sulfonamides. Bull. Soc. Chim. Fr. 3, 486–492 (1961)
Radulescu, D., Alexa, M.B.: Vapor pressure measurements from new dynamic and static methods. The applicability of the reaction isochore to the measurement of the latent heat of vaporization. Soc. Chim. Rom. 20A, 89–113 (1938)
Philippe, R., Jambon, C., Clechet, P.: Thermodynamic properties of dimethylsulfoxide + halomethane mixtures: II. Vapour pressures and excess thermodynamic functions. J. Chem. Thermodyn. 5, 431–444 (1973)
Sharma, B.R., Singh, P.P.: Excess Gibbs energies of mixing for some binary mixtures. J. Chem. Eng. Data 20, 360–363 (1975)
Kahlbaum, G.W.A.: The dependence of boiling temperature on the atmospheric pressure. Ber. Dtsch. Chem. Ges. 17, 1245–1262 (1884)
Boublik, T., Aim, K.: Heats of vaporization of simple non-spherical molecule compounds. Collect. Czech. Chem. Commun. 37, 3513–3521 (1972)
Paul, T., Schantz, K.: The boiling point as indicator of purity and a new apparatus for its determination without thermometer correction. Arch. Pharm. Weinheim 257, 87 (1919)
Manjeshwar, L.S., Aminabhavi, T.M.: Densities and viscosities of binary liquid mixtures containing bromoform at 45 °C. J. Chem. Eng. Data 33, 184–185 (1988)
Aminabhavi, T.M., Manjeshwar, L.S., Balundgi, R.H.: Viscosities of binary liquid mixtures. J. Chem. Eng. Data 32, 50–52 (1987)
Lecat, M.: New binary azeotropes: 7th List. Ann. Soc. Sci. Brux. Ser. B 47, 108–114 (1927)
Lecat, M.: Negative and other azeotropes. C. R. Hebd. Seances Acad. Sci. 217, 242–246 (1943)
Timmermans, J., Martin, F.: Study of the physical constants of twenty organic compounds. J. Chim. Phys. Phys. Chim. Biol. 25, 411–451 (1928)
Poling, B.E., Prausnitz, J.M., O’Connell, J.P.: The Properties of Gases and Liquids, 5th edn. McGraw–Hill Education (2001); ISBN: 9780070116825
Fan, C.L., Wang, L.S.: Vapor pressure of dimethyl phosphite and dimethyl methylphosphonate. J. Chem. Eng. Data 55, 479–481 (2010)
Wang, X., Dong, H., Zeng, Z., Wu, C.: Measurement and correlation of the saturated vapour pressure of vinyltriethoxysilane. J. Solution Chem. 44, 67–76 (2015)
Ihmels, C., Horstmann, S., Grybat, A.: Vapor pressures and vapor–liquid critical properties of four pentene isomers. J. Chem. Eng. Data 62, 2837–2841 (2017)
Xu, J., Li, S., Zeng, Z., Xue, W.: Heat capacity, density, vapor pressure, and enthalpy of vaporization of isoamyl DL-lactate. J. Chem. Eng. Data 64, 3793–3798 (2019)
Watson, K.M.: Thermodynamics of the liquid state. Ind. Eng. Chem. 35, 398–406 (1943)
Joback, K.G., Reid, R.C.: Estimation of pure-component properties from group-contributions. Chem. Eng. Commun. 57, 233–243 (1987)
Constantinou, L., Gani, R.: New group contribution method for estimating properties of pure compounds. AIChE J. 40, 1697–1710 (1994)
Constantinou, L., Gani, R., O’Connell, J.P.: Estimation of the acentric factor and the liquid molar volume at 298 K using a new group contribution method. Fluid Phase Equilib. 103, 11–22 (1995)
Marrero, J., Gani, R.: Group–contribution-based estimation of pure component properties. Fluid Phase Equilib. 183, 183–208 (2001)
Klincewicz, K.M., Reid, R.C.: Estimation of critical properties with group contribution methods. AIChE J. 30, 137–142 (1984)
Nannoolal, Y., Rarey, J., Ramjugernath, D., Cordes, W.: Estimation of pure component properties: Part 1. Estimation of the normal boiling point of non-electrolyte organic compounds via croup contributions and group interactions. Fluid Phase Equilib. 226, 45–63 (2004)
Nannoolal, Y., Rarey, J., Ramjugernath, D.: Estimation of pure component properties: Part 2. Estimation of critical property data by group contribution. Fluid Phase Equilib. 252, 1–27 (2007)
Sanghvi, R., Yalkowsky, S.H.: Estimation of the normal boiling point of organic compounds. Ind. Eng. Chem. Res. 45, 2856–2861 (2006)
Li, J., Xia, L., Xiang, S.: A new method based on elements and chemical bonds for organic compounds critical properties estimation. Fluid Phase Equilib. 417, 1–6 (2016)
Ghasemitabar, H., Movagharnejad, K.: Estimation of the normal boiling point of organic compounds via a new group contribution method. Fluid Phase Equilib. 411, 13–23 (2016)
Pascal, P.: Supposed formation of carbon from piperonyl derivatives. Bull. Soc. Chim. Fr. 37, 1043–1045 (1925)
Turner, W.E.S.: The molecular condition of some organic ammonium salts in bromoform. J. Chem. Soc. 101, 1923–1928 (1912)
Sherman, A., Sherman, J.: The coefficient of expansion of bromoform. J. Am. Chem. Soc. 50, 1119–1120 (1928)
Lagemann, R.T., Mcmillan, D.R., Woolf, W.E.: Temperature variation of ultrasonic velocity in liquids. J. Chem. Phys. 17, 369–373 (1949)
Perkin, W.H.: On the magnetic rotary polarisation of compounds in relation to their chemical consitution; with observations on the preparation and relative densities of the bodies examined. J. Chem. Soc. 45, 421–580 (1884)
Perkin, W.H.: The refractive power of ethylene dichloride is studied. J. Prakt. Chem. 32, 497–523 (1885)
Buhmann H.: Boiling point determination of bromoform. In: Dab: VI The physical constants of bromoform. Arch. Pharm. Weinheim 266, 123–126 (1928)
Thorpe, T.E.J.: On the relation between the molecular weights of substances and their specific gravities in the liquid state. Chem. Soc. 37, 141–143 (1880)
Friend, J.N., Hargreaves, W.D.: Viscosity at the boiling point, the rheochor. Philos. Mag. 34, 643–650 (1943)
Wolf, F., Sauerwald, F.: Surface tension measurements. Kolloid Z. 118, 1–10 (1950)
Gladstone, J.H.: XXXII—molecular refraction and dispersion of various substances. J. Chem. Soc. 59, 290–301 (1891)
Smyth, C.P., Rogers, E.H.: The dielectric polarization of liquids: IX. The electric moments of the alkyl halides and halogenated methanes. J. Am. Chem. Soc. 52, 2227–2240 (1930)
Patterson, T.S., Thomson, D.: XXXV—the influence of solvents on the rotation of optically active compounds. Part XI Ethyl tartrate in aliphatic halogen derivatives. J. Chem. Soc. 93, 355–371 (1908)
Kahlbaum, G.W.A.: Studies on vapor pressure measurements: II. Z. Phys. Chem. Stoechiom. Verwandschaftsl. 26, 577–658 (1898)
Oppenheimer, F.: Die Veränderung der Capillaritätskonstante des Quecksilbers Durch Zusatz Kleiner Mengen Alkali- und Erdalkalimetall. Z. Anorg. Allg. Chem. 171, 98–102 (1928)
Desreux, V.: A study of the parachor. Bull. Soc. Chim. Belg. 44, 249–287 (1935)
Earp, D.P., Glasstone, S.: Dielectric polarisation and molecular-compound formation in solution. J. Chem. Soc. 201, 709–1723 (1935)
Stevels, J.M.: The relation between refraction data and reactivity of halogenated methane derivatives. Trans. Faraday Soc. 33, 1381–1390 (1937)
Morgan, S.O., Yager, W.A.: Dielectric properties of organic components. Relation to chemical composition and physical structure. Ind. Eng. Chem. 32, 1519–1528 (1940)
Suhrmann, R., Klein, P.: The structure of the second CH harmonic oscillation and the determination of integral extinction equivalents in liquid aliphatic and aromatic hydrocarbons in the infrared spectrum. Z. Phys. Chem. B 50, 23–72 (1941)
Schaaffs, W.: Investigations on the velocity of sound and constitution I. The velocity of sound in organic liquids. Z. Phys. Chem. (Leipz.) 194, 28–38 (1944)
French, C.M., Trew, V.C.G.: Diamagnetic susceptibility of some alkyl and aryl halides. Trans. Faraday Soc. 41, 439–449 (1945)
Vogel, A.I.: Physical properties and chemical constitution: XVIII Miscellaneous compounds investigation of the so-called coordinate or dative link in esters of oxy acids and in nitro paraffins by molecular refractivity determinations. J. Chem. Soc. 1833–1855 (1948)
Parks, G.S.: Absorption by simultaneous diffusion and chemical reaction. Trans. Faraday Soc. 46, 300–304 (1950)
Auwers, K.V., Harres, L.: Zur Spektrochemie Aliphatischer Nitroverbindungen. Ber. Dtsch. Chem. Ges. 62b, 2287–2297 (1929)
Buchowski, H., Janaszewski, B.T.: Vapor pressure and excess free enthalpy of bromoform–2,2,4-trimethylpentane mixtures. J. Bull. Acad. Pol. Sci. Chim. 14, 403–407 (1966)
Dolezalek, F.S.: The theory of binary mixtures: VII The ethyl ether + bromoform mixture. Z. Phys. Chem. Stoechiom. Verwandschaftsl. 98, 395–400 (1921)
Trew, V.C.G.: Physical properties of mixtures of acetone and bromoform. Trans. Faraday Soc. 28, 509–514 (1932)
Tschamler, H., Richter, E., Wettig, F.: Binary liquid mixtures: XIII The miscibility of chlorine (β, β’-dichlorodiethylether) with halogenated hydrocarbon. Monatsh. Chem. 80, 856–863 (1949)
Singh, P.P., Malik, R., Maken, S., Acree, J.W.E., Zvaigzne, A.I.: Investigations of associated solutions. 12. Mole fraction vs. volume fraction based association constants for predicting excess molar enthalpies of acetone–bromoform–alkane mixtures. Thermochim. Acta 165, 113–127 (1990)
Aralaguppi, I.M., Aminabhavi, M.T., Balundgi, R.H., Joshi, S.S.: Thermodynamic interactions in mixtures of bromoform with hydrocarbons. J. Phys. Chem. 95, 5299–5308 (1991)
Aminabhavi, T.M., Raikar, S.K.: A study on mixing properties of binary mixtures of bromoform with aliphatic alcohols. J. Chem. Eng. Data 38, 310–319 (1993)
Blanco, S.T., Munoz, J., Velasco, I., Otin, S.: Excess molar enthalpies of binary mixtures containing mono- and polybromoalkanes at 298.15 K. J. Chem. Eng. Data 40, 605–606 (1995)
Aminabhavi, T.M., Patil, V.B.: Density, viscosity, refractive index, and speed of sound in binary mixtures of ethenylbenzene with N,N-dimethylacetamide, tetrahydrofuran, N,N-dimethylformamide, 1,4-dioxane, dimethyl sulfoxide, chloroform, bromoform, and 1-chloronaphthalene in the temperature interval (298.15–308.15) K. J. Chem. Eng. Data 43, 497–503 (1998)
Schmidt, R.L., Clever, H.L.: Thermodynamics of binary liquid mixtures by Rayleigh light scattering. J. Phys. Chem. 72, 1529–1536 (1968)
Aminabhavi, T.M., Aminabhavi, V.A., Joshi, S.S., Balundgi, R.H.: Excess properties of some binary liquid mixtures in the temperature range 298.15–313.15 K. Indian J. Technol. 29, 545–557 (1991)
Drucker, K., Kassel, R.: Fluidity of binary mixtures. Z. Phys. Chem. Stoechiom. Verwandschaftsl. 76, 367–384 (1911)
Kosuru, R.K., Aniya, V., Kumari, A., Chitturi, H.S., Sreeramoju, A., Thella, P.K., Satyavathi, B.: Measurement and correlation studies of phase equilibria and thermophysical properties of 4-tert-butylbenzaldehyde. J. Mol. Liq. 280, 11–17 (2019)
Hoffmann, D., Castier, M., Paredes, M.L.L., Mattedi, S.: Liquid phase density, sound speed, and vapor pressure of linear alkanes using the Mattedi–Tavares–Castier equation of state. Ind. Eng. Chem. Res. 58, 6767–6777 (2019)
Rackett, H.G.: Equation of state for saturated liquids. J. Chem. Eng. Data 15, 514–517 (1970)
Zheng, T., Fang, J., Xie, Q., Wu, Z., Lu, M., Xia, F., Deng, D., Nie, Y., Ji, J.: Measurement and correlation of the density, viscosity and vapor pressure of fatty acid 2-ethyhexyl esters. J. Chem. Thermodyn. 139, 243–250 (2019)
Nannoolal, Y., Rarey, J., Ramjugernath, D.: Estimation of purecomponent properties. Part 4: Estimation of the saturated liquid viscosity of non-electrolyte organic compounds via group contributions and group interactions. Fluid Phase Equilib. 281, 97–119 (2009)
Orrick, C., Erbar, J.H.: As Reported in R.C. Reid, J.M. Prausnitz, T.K. Sherwood, The Properties of Gases and Liquids, 3rd edn. McGraw–Hill, New York (1977)
Sastri, S.R.S., Rao, K.K.: A new group contribution method for predicting viscosity of organic liquids. Chem. Eng. J. 50, 9–25 (1992)
Power, P.P.: Inorganic Synthesis, vol. 37. Wiley, Hoboken (2018)
Reddy, A., Nagar, H., Satyavathi, B., Aniya, V.: Phase equilibria and thermophysical properties of dibromomethane: measurement and correlation studies. J. Mol. Liq. 306, 112917 (2020)
Aniya, V., Tangirala, R., Thella, P.K., Satyavathi, B.: Measurement and correlation studies of the saturated vapor pressure, density, refractive indices, and viscosity of methyl 4-tert-butylbenzoate. J. Chem. Eng. Data 62, 96–104 (2016)
Singh, M.: Survismeter, 2-In-1 for viscosity and surface tension measurement, an excellent invention for industrial proliferation of surface forces in liquids. Surf. Rev. Lett. 14, 973–983 (2007)
Nannoolal, Y., Rarey, J., Ramjugernath, D.: Estimation of pure component properties: Part 3. Estimation of vapor pressure of non-electrolyte organic compounds via group contribution and group interactions. Fluid Phase Equilib. 269, 117–133 (2008)
Layenz, J., Watson, I.: Enthalpies of vaporization of organic compounds. Acta Chem. Scand. 26, 3148–3152 (1972)
Trew, V.C.G., Spencer, J.F.: The magnetic susceptibility of binary systems of organic liquids. Proc. R. Soc. Lond. Ser. A 131, 209–213 (1931)
Aniya, V., De, D., Singh, A., Satyavathi, B.: Isobaric phase equilibrium of tert-butyl alcohol + glycerol at local and subatmospheric pressures, volumetric properties, and molar refractivity from 303.15 to 333.15 K of tert-butyl alcohol + glycerol, tert-butyl alcohol + water, and water + glycerol binary systems. J. Chem. Eng. Data 61, 1850–1863 (2016)
Beysens, D., Clamettes, P.: Temperature dependence of the refractive index of liquids: deviations from the Lorentz–Lorenz formula. J. Chem. Phys. 66, 766–771 (1977)
Riddick, J.A., Bunger, W.B., Sakano, T.K.: Organic Solvents: Physical Properties And Methods of Purification, 4th edn., vol. II. Wiley, New York (1986)
Pitzer, K.S., Kim, J.J.: Thermodynamics of electrolytes. IV. Activity and osmotic coefficients for mixed electrolytes. J. Am. Chem. Soc. 96, 5701–5707 (1974)
Acknowledgements
We thank the Director, CSIR-IICT (Ms. No. IICT/Pubs./2020/168) for providing all the required facilities to carry out this work.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
No potential conflict of interest is reported by the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
Rights and permissions
About this article
Cite this article
Naresh, K., Nagar, H. & Aniya, V. Thermodynamic Measurements and Correlation of Properties for Tribromomethane. J Solution Chem 50, 723–751 (2021). https://doi.org/10.1007/s10953-021-01076-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-021-01076-5