Abstract
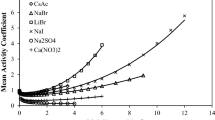
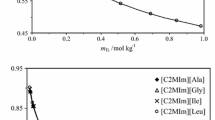
In the present study, a thermodynamic model is developed to predict the phase equilibrium of electrolyte solutions. In this model the Pitzer–Debye–Hückel equation was used to calculate the long-range contribution of the activity coefficient. To take into account the short-rang part of activity coefficient a new modified UNIQUAC-based model was developed. The model was applied for 18 binary electrolyte solutions. Results show that the model presented reproduces the osmotic coefficients of electrolyte solutions accurately to high concentration levels of salt. Comparison of standard deviation of the model and E-NRTL and E-UNIQUAC-NRF models was performed in the manuscript.


Similar content being viewed by others
References
Haghtalab, A., Peyvandi, K.: Electrolyte-UNIQUAC-NRF model for the correlation of the mean activity coefficient of electrolyte solutions. Fluid Phase Equilib. 281, 163–171 (2009)
Mazloumi, S.H.: Representation of activity and osmotic coefficients of electrolyte solutions using non-electrolyte Wilson–NRF model with ion-specific parameters. Fluid Phase Equilib. 388, 31–36 (2015)
Zhao, E., Yu, M., Sauvé, R.E., Khoshkbarchi, M.K.: Extension of the Wilson model to electrolyte solutions. Fluid Phase Equilib. 173, 161–175 (2000)
Maribo-Mogensen, B., Kontogeorgis, G.M., Thomsen, K.: Comparison of the Debye–Hückel and the mean spherical approximation theories for electrolyte solutions. Ind. Eng. Chem. Res. 51, 5353–5363 (2012)
Kontogeorgis, G.M., Maribo-Mogensen, B., Thomsen, K.: The Debye–Hückel theory and its importance in modeling electrolyte solutions. Fluid Phase Equilib. 462, 130–152 (2018)
Bromley, L.A.: Thermodynamic properties of strong electrolytes in aqueous solutions. AIChE 19, 313–320 (1973)
Pitzer, K.S.: Thermodynamics of electrolytes. I. Theoretical basis and general equations. J. Phys. Chem. 77, 268–277 (1973)
Bakhshi, H., Mobalegholeslam, P.: Phase equilibria calculations of electrolyte solutions containing water–polymer–salt using a new thermodynamic model, applicable in aqueous two phase systems. Fluid Phase Equilib. 434, 222–232 (2017)
Chen, C.C., Britt, H.I., Boston, J., Evans, L.: Local composition model for excess Gibbs energy of electrolyte systems. Part I: Single solvent, single completely dissociated electrolyte systems. AIChE 28, 588–596 (1982)
Haghtalab, A., Vera, J.: A nonrandom factor model for the excess Gibbs energy of electrolyte solutions. AIChE 34, 803–813 (1988)
Razavi, S.M., Haghtalab, A., Khanchi, A.R.: An electrolyte non-random-UNIQUAC model for thermodynamic modeling of binary and multicomponent aqueous electrolyte systems. J. Solution Chem. 48, 624–657 (2019)
Boukhalfa, N., Méniai, A.-H.: Assessment of a thermodynamic model for aqueous electrolyte systems. Int. J. Hydrogen Energy 43, 5358–5364 (2018)
Pitzer, K.S.: Electrolytes. From dilute solutions to fused salts. J. Am. Chem. Soc. 102, 2902–2906 (1980)
Bradley, D.J., Pitzer, K.S.: Thermodynamics of electrolytes. 12. Dielectric properties of water and Debye–Hückel parameters to 350. degree. C and 1 kbar. J. Phys. Chem. 83, 1599–1603 (1979)
Simonson, J.M., Pitzer, K.S.: Thermodynamics of multicomponent, miscible ionic systems: the system lithium nitrate–potassium nitrate–water. J. Phys. Chem. 90, 3009–3013 (1986)
Hill, T.: An Introduction to Statistical Thermodynamics, pp. 209–211. General Publishing Company, Toronto, Canada (1986)
Chen, C.C., Evans, L.B.: A local composition model for the excess Gibbs energy of aqueous electrolyte systems. AIChE 32, 444–454 (1986)
Pazuki, G., Nikookar, M.: A new local composition model for predicting of activity coefficient and solubility of amino acids and peptides in water. Biochem. Eng. J. 28, 44–49 (2006)
Messnaoui, B., Ouiazzane, S., Bouhaouss, A., Bounahmidi, T.: A modified electrolyte-Uniquac model for computing the activity coefficient and phase diagrams of electrolytes systems. Calphad 32, 566–576 (2008)
Hamer, W.J., Wu, Y.C.: Osmotic coefficients and mean activity coefficients of uni-univalent electrolytes in water at 25 °C. J. Phys. Chem. Ref. Data 1, 1047–1100 (1972)
Goldberg, R.N.: Evaluated activity and osmotic coefficients for aqueous solutions: thirty-six uni-bivalent electrolytes. J. Phys. Chem. Ref. Data 10, 671–764 (1981)
Acknowledgements
The authors acknowledge the funding support of Babol Noshirvani University of Technology for this study, through Grant Program No. BNUT/390058/97.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bakhshi, H., Mobalegholeslam, P. A Modification of UNIQUAC Model for Electrolyte Solutions Based on the Local Composition Concept. J Solution Chem 49, 1485–1496 (2020). https://doi.org/10.1007/s10953-020-01036-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-020-01036-5