Abstract
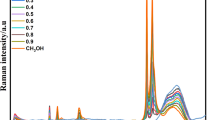
In this work, Raman spectroscopy is employed to investigate the effects of hydrophobic and hydrophilic groups on water structure. In aqueous methanol, ethanol, 1-propanol solutions, the addition of methylic groups (-CH3) raises the intensity ratio of the 3430 cm−1 sub-band to the 3220 cm−1 peak, and also increases the intensity of free OH vibrations. From our recent studies, this means that the increase of hydrophobic groups raises the ratio of DA (single donor-single acceptor) to DDAA (double donor-double acceptor) hydrogen bonding. Additionally, increasing the hydrophilic groups (–OH) as 1-propanol, 1,2-propanediol and 1,2,3-propanetriol solutions, also increases the ratio of DA to DDAA structural motifs, but decreases the free OH vibrations. The results of this study do not support the “iceberg” model of hydrophobic effects. Additionally, the dissolved hydrophobic and hydrophilic groups may have different effects on free OH vibrations.






Similar content being viewed by others
References
Frank, H.S., Evans, M.W.: Free volume and entropy in condensed systems III. Entropy in binary liquid mixtures; partial molal entropy in dilute solutions; structure and thermodynamics in aqueous electrolytes. J. Chem. Phys. 13, 507–532 (1945)
Kauzmann, W.: Some factors in the interpretation of protein denaturation. Adv. Protein. Chem. 13, 1–63 (1959)
Tanford, C.: Interfacial free energy and the hydrophobic effect. Proc. Natl. Acad. Sci. U.S.A. 76, 4175–4176 (1979)
Southall, N.T., Dill, K.A., Haymet, A.D.J.: A view of the hydrophobic effect. J. Phys. Chem. B 106, 521–533 (2002)
Chandler, D.: Interfaces and the driving force of hydrophobic assembly. Nature 437, 640–647 (2005)
Ball, P.: Water as an active constituent in cell biology. Chem. Rev. 108, 74–108 (2008)
Davis, J.G., Gierszal, K.P., Wang, P., Ben-Amotz, D.: Water structural transformation at molecular hydrophobic interfaces. Nature 491, 582–585 (2012)
Galamba, N.: Water’s structure around hydrophobic solutes and the iceberg model. J. Phys. Chem. B 117, 2153–2159 (2013)
Raschke, T.M., Levitt, M.: Nonpolar solutes enhance water structure within hydration shells while reducing interactions between them. Proc. Natl. Acad. Sci. U.S.A. 102, 6777–6782 (2005)
Rezus, Y.L.A., Bakker, H.J.: Observation of immobilized water molecules around hydrophobic groups. Phys. Rev. Lett. 99, 148301 (2007)
Grdadolnik, J., Merzel, F., Avbelj, F.: Origin of hydrophobicity and enhanced water hydrogen bond strength near purely hydrophobic solutes. Proc. Natl. Acad. Sci. U.S.A. 114, 322–327 (2017)
Qvist, J., Halle, B.: Thermal signature of hydrophobic hydration dynamics. J. Am. Chem. Soc. 130, 10345–10353 (2008)
Buchanan, P., Aldiwan, N., Soper, A.K., Creek, J.L., Koh, C.A.: Decreased structure on dissolving methane in water. Chem. Phys. Lett. 415, 89–93 (2005)
Lenton, S., Rhys, N.H., Towey, J.J., Soper, A.K., Dougan, L.: Temperature-dependent segregation in alcohol–water binary mixtures is driven by water clustering. J. Phys. Chem. B 122, 7884–7894 (2018)
Turner, J., Soper, A.K., Finney, J.L.: A neutron-diffraction study of tetramethylammonium chloride in aqueous solution. Mol. Phys. 70, 679–700 (1990)
Bakulin, A.A., Liang, C., Jansen, T.L., Wiersma, D.A., Bakker, H.J., Pshenichnikov, M.S.: Hydrophobic solvation: A 2D IR spectroscopic inquest. Acc. Chem. Res. 42, 1229–1238 (2009)
Lambeth, B.P., Junghans, C., Kremer, K., Clementi, C., Site, L.D.: Communication: On the locality of hydrogen bond networks at hydrophobic interfaces. J. Chem. Phys. 133, 221101 (2010)
Silvestrelli, P.L.: Are there immobilized water molecules around hydrophobic groups? Aqueous solvation of methanol from first principles. J. Phys. Chem. B 113, 10728–10731 (2009)
Juurinen, I., Pylkkanen, T., Sahle, C.J., Simonelli, L., Hämäläinen, K., Huotari, S., Hakala, M.: Effect of the hydrophobic alcohol chain length on the hydrogen-bond network of water. J. Phys. Chem. B. 118, 8750–8755 (2014)
Montagna, M., Sterpone, F., Guidoni, L.: Structural and spectroscopic properties of water around small hydrophobic solutes. J. Phys. Chem. B 116, 11695–11700 (2012)
Tsenkova, R.: Aquaphotomics: Dynamic spectroscopy of aqueous and biological systems describes peculiarities of water. J. Near Infrared Spectrosc. 17, 303–314 (2009)
Tsenkova, R.: Aquaphotomics: Water in the biological and aqueous world scrutinized with invisible light. Spectrosc. Eur. 22, 6–10 (2010)
Gowen, A.A., Amigo, J.M., Tsenkova, R.: Characterisation of hydrogen bond perturbations in aqueous systems using aquaphotomics and multivariate curve resolution-alternating least squares. Anal. Chim. Acta 759, 8–20 (2013)
Tsenkova, R., Munćan, J., Pollner, B., Kovacs, Z.: Essentials of aquaphotomics and its chemometrics approaches. Front. Chem. 6, 363 (2018)
Walrafen, G.E., Chu, Y.C.: Linearity between structural correlation length and correlated-proton Raman intensity from amorphous ice and supercooled water up to dense supercritical steam. J. Phys. Chem. 99, 11225–11229 (1995)
Khoshtariya, D.E., Zahl, A., Dolidze, T.D., Neubrand, A., Eldik, R.A.: Discrimination of diverse (pressure/temperature-dependent/independent) inherent sub-structures in liquid water (D2O) from difference vibrational spectroscopy. J. Phys. Chem. B 108, 14796–14799 (2004)
Li, F.B., Li, Z.L., Wang, Y., Wang, S.H., Wang, X.J., Sun, C.L., Men. Z.W.: A Raman spectroscopy study on the effects of intermolecular hydrogen bonding on water molecules absorbed by borosilicate glass surface. Spectrochim. Acta A: Mol. Spectrosc. 196, 317–322 (2018)
Kozlovskaya, E.N., Pitsevich, G.A., Malevich, A.E., Doroshenko, O.P., Pogorelov, V.E., Doroshenko, I.Y., Balevicius, V., Sablinskas, S., Kamnev, A.A.: Raman spectroscopic and theoretical study of liquid and solid water within the spectral region 1600–2300 cm−1. Spectrochim. Acta A: Mol. Spectrosc. 196, 406–412 (2018)
Sun, Q.: The Raman OH stretching bands of liquid water. Vib. Spectrosc. 51, 213–217 (2009)
Sun, Q.: Raman spectroscopic study of the effects of dissolved NaCl on water structure. Vib. Spectrosc. 62, 110–114 (2012)
Sun, Q.: Local statistical interpretation for water structure. Chem. Phys. Lett. 568–569, 90–94 (2013)
Aarnoutse, P., Westerhuis, J.: Quantitative Raman reaction monitoring using the solvent as internal standard. Anal. Chem. 77, 1228–1236 (2005)
Nah, S., Kim, D., Chung, H., Han. S. H., Yoon, M. Y.: A new quantitative Raman measurement scheme using teflon as a novel intensity correction standard as well as the sample container. J. Raman Spectrosc. 38, 475–482 (2007)
Park, S., Kim, M., Noh, J., Chung, H., Woo, Y., Lee, J., Kemper, M.S.: Reliable and fast quantitative analysis of active ingredient in pharmaceutical suspension using Raman spectroscopy. Anal. Chim. Acta 593, 46–53 (2007)
Sun, Q., Qin, C.: Raman OH stretching band of water as an internal standard to determine carbonate concentrations. Chem. Geol. 283, 274–278 (2011)
Sun, Q.: The physical origin of hydrophobic effects. Chem. Phys. Lett. 672, 21–25 (2017)
Sun, Q., Su, X.W., Cheng, C.B.: The dependence of hydrophobic interactions on solute size. Chem. Phys. 516, 199–205 (2019)
Sun, Q., Zhang, M.X., Cui, S.: The structural origin of hydration repulsive force. Chem. Phys. Lett. 714, 30–36 (2019)
Fraley, P.E., Rao, K.N.: High resolution infrared spectra of water vapor: ν1 and ν3 band of H216O. J. Mol. Spectrosc. 29, 348–365 (1969)
Ludwig, R.: The effect of hydrogen bonding on the thermodynamic and spectroscopic properties of molecular clusters and liquids. Phys. Chem. Chem. Phys. 4, 5481–5487 (2002)
Buck, U., Huisken, F.: Infrared spectroscopy of size-selected water and methanol clusters. Chem. Rev. 100, 3863–3890 (2000)
Hirabayashi, S., Yamada, K.M.: Infrared spectra of water clusters in krypton and xenon matrices. J. Chem. Phys. 122, 244501 (2005)
Pérez, C., Muckle, M.T., Zaleski, D.P., Seifert, N.A., Temelso, B., Shields, G.C., Kisiel, Z., Pate, B.H.: Structures of cage, prism, and book isomers of water hexamer from broadband rotational spectroscopy. Science 236, 897–901 (2012)
Martins, J.B.L., Politi, J.R.S., Garcia, E., Vilela, A.F.A., Gargano, R.: Theoretical study of CH4–CH4, CHF3–CH4, CH4–H2O, and CHF3–H2O dimers. J. Phys. Chem. A 113, 14818–14823 (2009)
Raghavendra, B., Arunan, E.: Hydrogen bonding with a hydrogen bond: The methane–water complex and the penta-coordinate carbon. Chem. Phys. Lett. 467, 37–40 (2008)
Dore, L., Cohen, R.C., Schmuttenmaer, C.A., Busarow, K.L., Elrod, M.J., Loeser, J.G., Saykally, R.J.: Far infrared vibration-rotation-tunneling spectroscopy and internal dynamics of methane–water: A prototypical hydrophobic system. J. Chem. Phys. 100, 863–876 (1994)
Fileti, E.E., Chaudhuri, P., Canuto, S.: Relative strength of hydrogen bond interaction in alcohol–water complexes. Chem. Phys. Lett. 400, 494–499 (2004)
Finneran, I.A., Carroll, P.B., Allodi, M.A., Blake, G.A.: Hydrogen bonding in the ethanol–water dimer. Phys. Chem. Chem. Phys. 17, 24210–24214 (2015)
Nedić, M., Wassermann, T.N., Larseny, R.W., Suhm, M.A.: A combined Raman and infrared jet study of mixed methanol–water and ethanol–water clusters. Phys. Chem. Chem. Phys. 13, 14050–14063 (2011)
Fileti, E.E., Castro, M.A., Canuto, S.: Calculations of vibrational frequencies, Raman activities and degrees of depolarization for complexes involving water, methanol and ethanol. Chem. Phys. Lett. 452, 54–58 (2008)
Galano, A., Narciso-Lopez, M., Francisco-Marquez, M.: Water complexes of important air pollutants: Geometries, complexation energies, concentrations, infrared spectra, and intrinsic reactivity. J. Phys. Chem. A 114, 5796–5809 (2010)
Sun, Q.: The single donor-single acceptor hydrogen bonding structure in water probed by Raman spectroscopy. J. Chem. Phys. 132, 054507 (2010)
Crupi, V., Interdonato, S., Longo, F., Migliardo, P., Venuti. V.: A new insight on the hydrogen bonding structures of nanoconfined water: A Raman study. J. Raman Spectrosc. 39, 244–249 (2008)
Scatena, L.F., Brown, M.G., Richmond, G.L.: Water at hydrophobic surfaces: Weak hydrogen bonding and strong orientation effects. Science 292, 908–912 (2001)
Jubb, A.M., Hua, W., Allen, H.C.: Organization of water and atmospherically relevant ions and solutes: Vibrational sum frequency spectroscopy at the vapor/liquid and liquid/solid interfaces. Acc. Chem. Res. 45, 110–119 (2012)
Shen, Y.R., Ostroverkhov, V.: Sum-frequency vibrational spectroscopy on water interfaces: Polar orientation of water molecules at interfaces. Chem. Rev. 106, 1140–1154 (2006)
Richmond, G.L.: Molecular bonding and interactions at aqueous surfaces as probed by vibrational sum frequency spectroscopy. Chem. Rev. 102, 2693–2724 (2002)
Sun, Q., Guo, Y.: Vibrational sum frequency generation spectroscopy of the air/water interface. J. Mol. Liq. 213, 28–32 (2016)
Tian, C.S., Shen, Y.R.: Sum-frequency vibrational spectroscopic studies of water/vapor interfaces. Chem. Phys. Lett. 470, 1–6 (2009)
Ji, N., Ostroverkhov, V., Tian, C.S., Shen, Y.R.: Characterization of vibrational resonances of water–vapor interfaces by phase-sensitive sum-frequency spectroscopy. Phys. Rev. Lett. 100, 096102 (2008)
Acknowledgements
The reviewers and editor are greatly appreciated for providing good suggestions to revise the paper. This work is supported by the National Natural Science Foundation of China (Grant No. 41773050).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sun, Q. The Effects of Dissolved Hydrophobic and Hydrophilic Groups on Water Structure. J Solution Chem 49, 1473–1484 (2020). https://doi.org/10.1007/s10953-020-01035-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-020-01035-6