Abstract
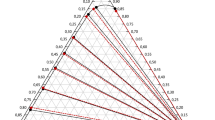
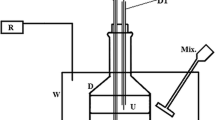
Liquid–liquid equilibrium (LLE) data for the ternary systems composed of water, C1–C4 monocarboxylic acids, and dibutyl ether were determined at three different temperatures (293.15, 303.15, and 313.15 K) and atmospheric pressure. The experimental solubility curves and mutual solubilities of (water + dibutyl ether) were determined by the cloud-point method, whereas the tie-lines of conjugate phases were obtained via GC analysis. The examined C1–C4 monocarboxylic acids are valuable components for estimating the effect of acid structure on LLE. The separation factor calculated from experimental LLE data is indicative of the ability of the solvent to effectively recover the acid. Equilibrium distribution of C1–C4 acids is better for more structured propionic and butyric acids as compared to less structured formic and acetic acids, and the general ranking with respect to the separation factor (S) follows the order: C4 > C3 > C2 > C1, and T293.15 < T303.15 ≤ T313.15. The LLE data were predicted with the group-contribution method A-UNIFAC with the newly estimated parameters, which shows an appropriate consistency with the experimental data.




Similar content being viewed by others
References
Bizzari, S., Ishikawa, Y.: CEH Report: Formic Acid. SRI, Menlo Park, CA (2001)
Grasemann, M., Laurenczy, G.: Formic acid as a hydrogen source – recent developments and future trends. Energy Environ. Sci. 5, 8171–8181 (2012)
Lee, L.S., Lin, C.H.: Phase behaviors of water + acetic acid + methyl acetate + p-xylene mixture at 101.32 kPa. Open Thermodyn. J. 2, 44–52 (2008)
Boyaval, P., Corre, C.: Production of propionic acid. Lait. 75, 453–461 (1995)
Dwidar, M., Park, J.Y., Mitchell, R.J., Sang, B.I.: The future of butyric acid in industry. Sci. World J. (2012). https://doi.org/10.1100/2012/471417
Zhang, C., Yang, H., Yang, F., Ma, Y.: Current progress on butyric acid production by fermentation. Curr. Microbiol. 59(6), 656–663 (2009)
Langford, R.E., Heric, E.L.: Furfural–water–formic acid system at 25° and 35 °C. J. Chem. Eng. Data 17, 87–89 (1972)
Bilgin, M., Birman, I.: Liquid phase equilibria of (water + formic acid + diethyl carbonate or diethyl malonate or diethyl fumarate) ternary systems at 298.15 K and atmospheric pressure. Fluid Phase Equilib. 302, 249–253 (2011)
Ghanadzadeh Gilani, H., Azadian, M.: Tie-line data for water–formic acid–1-decanol ternary system at T = 298.2, 303.2, 313.2, and 323.2 K. Thermochim. Acta 547, 141–145 (2012)
Ghanadzadeh Gilani, H., Noury, S., Asan, S.: Phase equilibrium data for aqueous solutions of formic acid with 2-ethyl-1-hexanol at T = (298.2, 308.2, 318.2, and 328.2) K. Korean J. Chem. Eng. 30, 1289–1294 (2013)
Timedjeghdine, M., Hasseine, A., Binous, H., Bacha, O., Attarakih, M.: Liquid–liquid equilibrium data for water + formic acid and solvent (butyl acetate, ethyl acetate, and isoamyl alcohol) at T = 291.15 K. Fluid Phase Equilib. 415, 51–57 (2016)
Senol, A., Bilgin, M., Baslioglu, B., Vakili-Nezhaad, G.: Modeling phase equilibria of ternary systems (water + formic acid + ester or alcohol) through UNIFAC-original, SERLAS, NRTL, NRTL-modified, and three-suffix Margules: parameter estimation using genetic algorithm. Fluid Phase Equilib. 429, 254–265 (2016)
Miao, X., Zhang, H., Wang, T., He, M.: Liquid–liquid equilibria of the ternary system water + acetic acid + methyl tert-butyl ether. J. Chem. Eng. Data 52, 789–793 (2007)
Laiadi, D., Hasseine, A., Merzougui, A.: Homotopy method to predict liquid–liquid equilibria for ternary mixtures of (water + carboxylic acid + organic solvent). Fluid Phase Equilib. 313, 114–120 (2012)
Lei, F., Wang, Q., Gong, X., Shen, B., Mao, B., Ye, X.: Liquid−liquid equilibrium for ternary system water + cyclohexane + acetic acid at (303.2 to 333.2) K. J. Chem. Eng. Data 58, 3489–3493 (2013)
Cháfer, A., Lladosa, E., de la Torre, J., Burguet, M.C.: Study of liquid–liquid equilibrium of the systems isobutyl acetate + acetic acid + water and isobutyl alcohol + acetic acid +water at different temperatures. Fluid Phase Equilib. 271, 76–81 (2008)
Aljimaz, A.S., Fandary, M.S.H., Alkandary, J.A., Fahim, M.A.: Liquid–liquid equilibria of the ternary system water + acetic acid + 1-heptanol. J. Chem. Eng. Data 45, 301–303 (2000)
Fahim, M.A., Al-Muhtaseb, S.A., Al-Nashef, I.M.: Phase equilibria of the ternary system water + acetic acid + 1-pentanol. J. Chem. Eng. Data 41, 562–565 (1996)
Arce, A., Blanco, A., Souza, P., Vidal, I.: Liquid-liquid equilibria of the ternary system water + propanoic acid + methyl isobutyl ketone at various temperatures. J. Chem. Eng. Data 38, 201–203 (1993)
Ghanadzadeh, H., Ghanadzadeh, A., Moeina, M., Shekarsaraeea, S., Jamshidia, Y.: Binodal curves and tie line data of the water–propionic acid–iso-butyl acetate at T = (298.2, 308.2, 318.2, and 328.2) K. Thermochim. Acta 540, 116–122 (2012)
Ghanadzadeh, H., Ghanadzadeh, A., Bahrpaima, Kh, Seyed Saadat, S.L.: (Liquid + liquid) equilibria of (water + propionic acid + 2-ethyl-1-hexanol): experimental data and correlation. J. Chem. Thermodyn. 40, 879–884 (2008)
Ozmen, D.: (Liquid + liquid) equilibria of (water + propionic acid + methyl isoamyl ketone or diisobutyl ketone or ethyl isoamyl ketone) at T = 298.2 K. Fluid Phase Equilib. 250, 70–75 (2006)
Çehreli, S., Tatlı, B., Bağman, P.: (Liquid + liquid) equilibria of (water + propionic acid + cyclohexanone) at several temperatures. J. Chem. Thermodyn. 37, 1288–1293 (2005)
Çehreli, S., Özmen, D., Tatlı, B.: (Liquid + liquid) equilibria of (water + propionic acid + diethyl phthalate) at several temperatures. J. Chem. Thermodyn. 37, 1144–1150 (2005)
Senol, A.: Liquid–liquid equilibria for ternary systems of (water + carboxylic acid + 1-octanol) at 293.15 K: modeling phase equilibria using a solvatochromic approach. Fluid Phase Equilib. 227, 87–96 (2005)
Luo, L., Liu, D., Li, L., Chen, Y.: Phase equilibria of (water + propionic acid or butyric acid + 2-methoxy- 2-methylpropane) ternary systems at 298.2 K and 323.2 K. Fluid Phase Equilib. 403, 30–35 (2015)
Ghanadzadeh Gilani, A., Ghanadzadeh Gilani, H., Amouzadeh, F.: Tie line data for the (water + butyric acid + n-butyl alcohol or amyl alcohol) at T = (298.2, 308.2, and 318.2) K and (water + butyric acid + isoamyl alcohol) at T = 298.2 K. J. Chem. Thermodyn. 71, 103–111 (2014)
Ghanadzadeh, H., Ghanadzadeh, A., Asgharzadeh, S., Moghadam, M.: Measurement and correlation of phase equilibrium data of the mixtures consisting of butyric acid, water, cyclohexanone at different temperatures. J. Chem. Thermodyn. 47, 288–294 (2012)
Senol, A.: Liquid–liquid equilibria for the system (water + carboxylic acid + chloroform): thermodynamic modelling. Fluid Phase Equilib. 243, 51–56 (2006)
Chen, Y., Wang, Y., Zhou, S., Chen, H., Liu, D., Li, L.: Liquid phase equilibrium of the ternary systems, water + propionic or butyric acid + mesityl oxide, at (298.2 and 323.2) K. J. Chem. Thermodyn. 111, 72–79 (2017)
Sandler, S.I.: Chemical, Biochemical, and Engineering Thermodynamics. John Wiley & Sons, New York (2006)
Tassin, N.G., Mascietti, V.A., Cismondi, M.: Phase behavior of multicomponent alkane mixtures and evaluation of predictive capacity for the PR and RKPR EoS's. Fluid Phase Equilib. 480, 53–65 (2019)
Trofimova, M., Sadaev, A., Samarov, A., Toikka, M., Toikka, A.: Solubility, liquid–liquid equilibrium and critical states for the quaternary system formic acid–ethanol–ethyl formate–water at 298.15 K and 308.15 K. Fluid Phase Equilib. 485, 111–119 (2019)
Ferreira, O., Macedo, E.A., Bottini, S.B.: Extension of the A-UNIFAC model to mixtures of cross- and self-associating compounds. Fluid Phase Equilib. 227, 165–176 (2005)
Mengarelli, A.C., Brignole, E.A., Bottini, S.B.: Activity coefficients of associating mixtures by group contribution. Fluid Phase Equilib. 163, 195–207 (1999)
Lide, D.R. (ed.): CRC Handbook of Chemistry and Physics. Internet Version. CRC Press/Taylor and Francis, Boca Raton, FL (2009)
Kumari, A., Sandeepa, K., Kumar, T.P., Satyavathi, B.: Solubility, thermodynamic properties, and derived excess properties of benzoic acid in (acetic acid + water) and (acetic acid + toluene) binary mixtures. J. Chem. Eng. Data 61, 67–77 (2016)
Suarez, F., Romero, C.M.: Apparent molar volume and surface tension of dilute aqueous solutions of carboxylic acids. J. Chem. Eng. Data 56, 1778–1786 (2011)
Wertheim, M.S.: Fluids with highly directional attractive forces. I. Statist. Thermodyn. J. Stat. Phys. 35, 19–34 (1984)
Fredenslund, A., Gmehling, J., Michelsen, M.L., Rasmussen, P., Prausnitz, J.M.: Computerized design of multicomponent distillation columns using the unifac group contribution method for calculation of activity coefficients. Ind. Eng. Chem. Process Des. Dev. 16, 450–462 (1977)
Ferreira, O., Brignole, E.A., Macedo, E.A.: Phase equilibria in sugar solutions using the A-UNIFAC model. Ind. Eng. Chem. Res. 42, 6212–6222 (2003)
Montañés, F., Olano, A., Ibáñez, E., Fornari, T.: Modeling solubilities of sugars in alcohols based on original experimental data. AIChE J. 53, 2411–2418 (2007)
Andreatta, A.E., Casa, L.M., Hegel, P., Bottini, S.B., Brignole, E.A.: Phase equilibria in ternary mixtures of methyl oleate, glycerol, and methanol. Ind. Eng. Chem. Res. 47, 5157–5164 (2008)
Sánchez, F.A., Ille, Y., Dahmen, N., Pereda, S.: GCA-EOS extension to mixtures of phenol ethers and derivatives with hydrocarbons and water. Fluid Phase Equilib. 490, 13–21 (2019)
Góral, M., Mączyński, A., Oracz, P.: Recommended liquid–liquid equilibrium data. part 5. Ether–water systems. J. Phys. Chem. Ref. Data 36, 1399–1415 (2007)
Stephenson, R.M.: Mutual solubilities: water–ketones, water–ethers, and water–gasoline–alcohols. J. Chem. Eng. Data 37, 80–95 (1992)
Park, S.J., Hwang, I.C., Kwak, H.Y.: Binary liquid−liquid equilibrium (LLE) for dibutyl ether (DBE) + water from (288.15 to 318.15) K and ternary LLE for systems of DBE + C1 ∼ C4 alcohols + water at 298.15 K. J. Chem. Eng. Data 53, 2089–2094 (2008)
Arlt, W., Gmehling, J., Onken, U.: Vapor–Liquid Equilibrium Data Collection. Aldehydes and Ketones Ethers, Vol. I; Part 3+4. DECHEMA, Frankfurt (1979)
Meehan, G.F., Murphy, N.F.: A new correlation for binary systems with an associating component. Chem. Eng. Sci. 20, 757–769 (1965)
Miyamoto, S., Nakamura, S., Iwai, Y., Arai, Y.: Measurement of isothermal vapor−liquid equilibria for binary and ternary systems containing monocarboxylic acid. J. Chem. Eng. Data 46, 1225–1230 (2001)
Gmehling, J., Rasmussen, P., Fredenslund, A.: Vapor-liquid equilibriums by UNIFAC group contribution. Revision and extension. 2. Ind. Eng. Chem. Process Des. Dev. 21, 118–127 (1982)
Ito, T., Yoshida, F.: Vapor-liquid equilibria of water-lower fatty acid systems: water-formic acid, water acetic acid and water-propionic acid. J. Chem. Eng. Data 8, 315–320 (1963)
Gmehling, J., Onken, U.: Vapor-Liquid Equilibrium Data Collection. Aqueous–Organic Systems, DECHEMA Chemistry Data Series, Vol. I, Part 1, Germany (1977)
Gmehling, J., Onken U., Grenzheuser, P.: Vapor–Liquid Equilibrium Data Collection. Carboxilic Acid, Anhydridus, Ester. DECHEMA Chemistry Data Series, Vol. I, Part 5, Germany (1980)
Fredenslund, A., Jones, R.L., Prausnitz, J.M.: Group-contribution estimation of activity coefficients in nonideal liquid mixtures. AIChE J. 21, 1086–1099 (1975)
Gros, H.P., Bottini, S., Brignole, E.A.: A group contribution equation of state for associating mixtures. Fluid Phase Equilib. 116, 537–544 (1996)
Fu, Y.H., Sandler, S.I., Orbey, H.: A Modified UNIQUAC model that includes hydrogen bonding. Ind. Eng. Chem. Res. 34, 4351–4363 (1995)
Fu, Y.H., Orbey, H., Sandler, S.I.: Prediction of vapor−liquid equilibria of associating mixtures with UNIFAC models that include association. Ind. Eng. Chem. Res. 35, 4656–4666 (1996)
Huang, S.H., Radosz, M.: Equation of state for small, large, polydisperse, and associating molecules. Ind. Eng. Chem. Res. 29, 2284–2294 (1990)
Michelsen, M.L., Hendriks, E.M.: Physical properties from association models. Fluid Phase Equilib. 180, 165–174 (2001)
Tan, S.P., Adidharma, H., Radosz, M.: Generalized procedure for estimating the fractions of nonbonded associating molecules and their derivatives in thermodynamic perturbation theory. Ind. Eng. Chem. Res. 43, 203–208 (2004)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix: A-UNIFAC
Appendix: A-UNIFAC
Mengarelli et al. [35] modified the original UNIFAC model presented by Fredenslund et al. [55] to take into account the associative effects in the calculation of activity coefficients. This model called A-UNIFAC adds an associative (assoc) term based on Wertheim's theory [39] to the original combinatorial (comb) and residual (res) expressions [40], and applies an approach to group contributions [56].
The non-ideal behavior is described as the sum of three independent contributions to the excess Gibbs energy function:
which in terms of the activity coefficient of a compound i in a multicomponent solution is:
The combinatorial and residual contributions are the same as in the UNIFAC model [55]. The associative contribution to the activity coefficient γassoc is derived from the association residual Helmholtz energy [57,58,59], which is function of the fraction of non-bonded sites in the solution, \(X^{{A_{k} }}\) and in pure component i, \(X_{i}^{{A_{k} }}\). It can be written as:
where Nk is the number of moles of associating group k, \(\nu_{k}^{i}\) is the number of associative groups of type k present in the compound i and the last term is the partial derivative of \(X^{{A_{k} }}\) with regards to the number of moles ni of this compound. The summation is extended to all NGA associating groups and Ak associating sites.
The fraction of non-bonded sites, \(X^{{A_{k} }}\) and the fraction non-bonded sites in the pure component i, \(X_{i}^{{A_{k} }}\) are functions of the associating group density in the mixture \(\rho_{j}\) and in the pure component i \((\rho_{j} )_{i}\), respectively, together with the association strength \(\mathop \Delta \nolimits^{{A_{k} B_{j} }}\) between site A of group k and site B of group j. These expressions, respectively, are:
where the sum is extended to all the NGA associative groups and Bj active sites of each group.
The density of the associative group j in the mixture \(\rho_{j}\) and in the pure component i \((\rho_{j} )_{i}\) are dimensionless and are calculated through the standardized Van der Waals volumes ri of each component of the mixture:
and
where NC is the total number of components of the mixture, \(\nu_{j}^{i}\) is the number of times that the associative group j is present in the molecule i and xi represents the molar fractions of the component i in the mixture.
The association strength \(\mathop \Delta \nolimits^{{A_{k} B_{j} }}\) is a function of the temperature and the energy \(\mathop \varepsilon \nolimits^{{A_{k} B_{j} }}\)(K) and volume \(\mathop \kappa \nolimits^{{A_{k} B_{j} }}\) (cm3·mol–1) of association, respectively, are represented by a model of association potential of the square-well type:
The evaluation of \(\gamma_{i}^{{{\text{assoc}}}}\) in Eq. 3 requires the calculation of partial derivatives of the fraction of non-bonded groups. However, Michelsen and Hendriks [60] applied a minimization procedure that simplifies calculations by avoiding such derivatives. Appling this approach, then Eq. 3 is reduced to:
The final expression of \(\gamma_{i}^{{{\text{assoc}}}}\) in a conventional procedure depends on the amount and type of association sites and species present in the solution with a computation of problem-dependent association effects. However, with the generalized procedure proposed by Tan et al. [61] it is possible to calculate the fraction of non-bonded molecules applicable to all associating systems, regardless of the number and type of associating sites and the number of components in the mixture.
Rights and permissions
About this article
Cite this article
Yalın, İ., Çehreli, S., Andreatta, A.E. et al. Phase Equilibria of Ternary Liquid–Liquid Systems (Water + C1–C4 Monocarboxylic Acids + Dibutyl Ether) at Three Different Temperatures: Modeling with A-UNIFAC. J Solution Chem 49, 1009–1028 (2020). https://doi.org/10.1007/s10953-020-01006-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-020-01006-x