Abstract
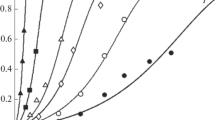
Magnetite is a major corrosion product of carbon steel that forms deposits in the steam generators in water-cooled nuclear reactors. Sulfate is present as an impurity in the steam generator feed water and accumulates in the magnetite deposits, leading to formation of acidic crevices, corrosion and stress corrosion cracking of steam generator tubing. Reliable adsorption data are required to understand material degradation of steam generator tubing. Sulfate adsorption onto magnetite has been studied at temperatures from 25 to 300 °C as a function of pH, and chloride and sulfate concentrations. The results show that adsorption decreases with increasing pH and ionic strength, and adsorption followed the Langmuir adsorption isotherm. Overall, sulfate adsorption onto magnetite is endothermic and the enthalpy of adsorption depends on the pH and ionic strength of the solutions. The adsorption behavior of sulfate onto magnetite is explained through outer-sphere and inner-sphere adsorption.













Similar content being viewed by others
Notes
CANDU®: CANada Deuterium Uranium, registered trademark of Atomic Energy of Canada Ltd.
pH values of all solutions were measured at 25 °C.
The pH values at 300 °C calculated from solution thermodynamics are 2.70, 3.35 and 3.68, respectively.
References
Sara, B., Combrade, P., Erre, R., Benoit, R., Le Calvar, M.: Chemistry of sulphur in high temperature water reduction of sulphates. In: Proceedings of the 5th International Symposium on Environmental Degradation of Materials in Nuclear Power Systems–Water Reactors, Monterey, CA, USA (1991)
Boursier, J.M., Dupin, M., Gossot, P., Rouillon, Y.: Contribution of surface analysis to the understanding of the degradation process. In: Proceedings of the 9th International Symposium on Environmental Degradation of Materials in Nuclear Power Systems-Water Reactors, New Port Beach, CA, USA (1999)
Wijnja, H., Schulthess, C.P.: Vibrational spectroscopy study of selenate and sulphate adsorption mechanisms on Fe and Al (hydr)oxide surfaces. J. Colloid Interface Sci. 229, 286–297 (2000)
Fukushi, K., Aoyama, K., Yang, C., Kitadai, N., Nakashima, S.: Surface complexation modeling for sulphate adsorption with in situ infrared spectroscopic observations. Appl. Geochem. 36, 92–103 (2013)
Person, P., Lӧvgren, L.: Potentiometric and spectroscopic studies of sulphate complexation at the goethite–water interface. Geochim. Cosmochim. Acta 60, 2789–2799 (1996)
Yates, D.E., Healy, T.W.: Mechanism of anion adsorption at the ferric and chromic oxide/water interfaces. J. Colloid Interface Sci. 52, 222–228 (1975)
Charlet, L., Dise, N., Stumm, W.: Sulphate adsorption on a viable charge soil and on reference materials. Agr. Ecosyst. Environ. 47, 87–102 (1993)
Juang, R.S., Wu, W.L.: Adsorption of sulphate and copper(II) on goethite in relation to the charges of zeta potentials. J. Colloid Interface Sci. 249, 22–29 (2002)
Geelhoed, J.S., Hiemstra, T., van Riemsdijk, W.H.: Phosphate and sulphate adsorption on goethite: single anion and competitive adsorption. Geochim. Cosmochim. Acta 61, 2389–2396 (1997)
Rahnemaie, R., Hiemstra, T., van Riemsdijk, W.H.: Inner- and outer-sphere complexation of ions at the goethite–solution interface. J. Colloid Interface Sci. 297, 379–388 (2006)
Fukushi, K., Sverjensky, D.A.: A surface complexation model for sulfate and selenate on iron oxides consistent with spectroscopic and theoretical molecular evidence. Geochim. Cosmochim. Acta 71, 1–24 (2007)
Pochard, I., Denoyel, R., Couchot, P., Foissy, A.: Adsorption of barium and calcium chloride onto negatively charged & #x03B1;-Fe2O3 particles. J. Colloid Interface Sci. 255, 27–35 (2002)
Ridley, M.K., Machesky, M.L., Wesolowski, D.J., Palmer, D.A.: Calcium adsorption at the rutile–water Interface: a potentiometric study in NaCl media to 250 °C. Geochim. Cosmochim. Acta 63, 3087–3096 (1999)
Qiu, L., Snaglewski, A.P.: Lithium adsorption on magnetite, lepidocrocite and maghemite at elevated temperatures. Nucl. Sci. Eng. 179, 199–210 (2015)
Qiu, L., Guzonas, D.A., Webb, D.G.: Zirconium dioxide solubility in high temperature aqueous solutions. J. Solution Chem. 38, 857–867 (2009)
Kersten, M., Vlasova, N.: The influence of temperature on selenate adsorption by goethite. Radiochim. Acta 101, 413–419 (2013)
Kumari, M., Pittman Jr., C.U., Mohn, D.: Heavy metals [chromium(VI) and lead(II)] removal from water using mesoporous magnetite (Fe3O4) nanospheres. J. Colloid Interface Sci. 442, 120–132 (2015)
Hao, L., Ouyang, T., Lai, L., Liu, Y.X., Chen, S., Hu, H., Chang, C.T., Wang, J.J.: Temperature effects on arsenate adsorption onto goethite and its preliminary application to arsenate removal from simulative geothermal water. RSC Adv. 4, 51984–51990 (2014)
Cornell, R.M., Schwertmann, U.: The Iron Oxides: Structure, Properties, Reactions, Occurrences and Uses. Wiley, New York (2000)
Ramachandran, V.S., Paroli, R.M., Beaudoin, J.J., Delgado, A.H.: Handbook of Thermal Analysis of Construction Materials. Noyes, New York (2003)
Zayani, L., Chehimi, D.B.H.: Synthesis and thermal behaviour of Na2SO4·MgSO4·4H2O. J. Therm. Anal. Calorim. 123, 1205–1211 (2016)
Roonasi, P., Holmgren, A.: An ATR-FTIR study of sulphate sorption on magnetite: rate of adsorption, surface speciation and effect of calcium ions. J. Colloid Interface Sci. 333, 27–32 (2009)
Ali, M.A., Dzombak, D.A.: Competitive sorption of simple organic acids and sulphate on goethite. Environ. Sci. Technol. 30, 1061–1071 (1996)
Kosmulski, M.: Compilation of PZC and IEP of sparingly soluble metal oxides and hydroxides from literature. Adv. Colloid Interface Sci. 152, 14–25 (2009)
Mansour, C., Lefèvre, G., Pavageau, E.M., Catalette, H., Fèdoroff, M., Zanna, M.S.: Sorption of sulphate ions onto magnetite. J. Colloid Interface Sci. 331, 77–82 (2009)
Peak, D., Ford, R.G., Sparks, D.L.: An in situ AFR-FTIR investigation of sulphate bonding mechanism on goethite. J. Colloid Interface Sci. 218, 289–299 (1999)
Rietra, R.P.J.J., Hiemstra, T., Riemsdijk, H.V.: Sulphate adsorption on goethite. J. Colloid Interface Sci. 218, 511–521 (1999)
Bentiss, F., Lebrini, M., Lagrenée, M.: Thermodynamic characterization of metal dissolution and inhibitor adsorption process in mild steel/2.5-bis(n-thienyl)-1.3,4-thiadiazoles/hydrochloric acid system. Corros. Sci. 47, 2915–2931 (2005)
Saeed, M.M.: Adsorption profile and thermodynamic parameters of the preconcentration of Eu(III) on 2-thenoyltrifluoroacetone loaded polyurethane (PUR) foam. J. Radioanal. Nucl. Chem. 256, 73–80 (2003)
Wesolowski, D.J., Palmer, D.A., Machesky, M.L., Anovitz, L.M., Hyde, K.E., Hayashi, K.I.: Solubility and surface charge characterization of magnetite in hydrothermal solutions. In: Tremaine, P.R., Hill, P.G., Irish, D.E., Balakrishnan, P.V. (eds.) Steam, Water, and Hydrothermal Systems: Physics and Chemistry Meeting the Needs of Industry, NRC Research Press, Ottawa, pp. 754–763 (2000)
Akcay, M.: Characterization and adsorption properties of tetrabutylammonium montmorillonite (TBAM) clay: thermodynamic and kinetic calculations. J. Colloid Interface Sci. 296, 16–21 (2006)
Hayes, K.F., Papelis, C., Leckie, J.O.: Modeling ionic strength on anion adsorption at hydrous oxide/solution interface. J. Colloid Interface Sci. 125, 717–726 (1988)
Palmer, D.A., Prini, R.F., Harvey, A.H.: Aqueous Systems at Elevated Temperatures and Pressures: Physical Chemistry in Water, Steam, and Hydrothermal Solutions. Elsevier Ltd., Amsterdam (2004)
Machesky, M.L.: Influence of temperature on ion adsorption by hydrous metal oxides (Chap. 22). Chemical Modeling of Aqueous Systems II. American Chemical Society, Washington, D.C. (1990)
Wesolowski, D.J., Machesky, M.L., Palmer, D.A., Anovitz, L.M.: Magnetite surface charge studies to 290 °C from in situ pH titrations. Chem. Geol. 167, 193–229 (2000)
Acknowledgements
This work was financially supported by Federal Science and Technology Programs at Canadian Nuclear Laboratories through the work package FST-51100.01.11.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Qiu, L., Burton, G.R., Rousseau, S. et al. Kinetics and Thermodynamics of Sulfate Adsorption on Magnetite at Elevated Temperatures. J Solution Chem 48, 1488–1502 (2019). https://doi.org/10.1007/s10953-019-00933-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-019-00933-8