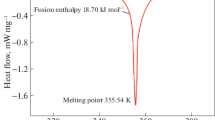
Abstract
The equilibrium solubilities of malonic acid in 2-propanol and ethyl acetate mono solvents, as well as in (2-propanol + ethyl acetate) binary solvent mixtures, were determined from 278.15 to 313.15 K. The obtained solubility data were correlated using thermodynamic models including the modified Apelblat equation, λh equation, NRTL model, GSM model and the modified Jouyban–Acree model. The dissolution mechanism of malonic acid in (2-propanol + ethyl acetate) solvent mixtures was interpreted theoretically. The inverse Kirkwood–Buff integrals method was applied and it was found that the value of δx1,3 is negative in high ethyl acetate fractions, but becomes positive at 2-propanol mole fractions greater than 0.60 at 298.15 K. In addition, molecular dynamic simulations were carried out to characterize the intermolecular interactions using the radial distribution function.








Similar content being viewed by others
References
Li, T., Lorenz, H., Seidel-Morgenstern, A.: Solubility study and thermal stability analysis of calcium propionate. Chem. Eng. Technol. 40, 1221–1230 (2017)
Sangwal, K., Mielniczek-Brzóska, E., Barylska, S.: Solubility of ammonium oxalate in water–acetone mixtures and metastable zone width of their solutions. Chem. Eng. Res. Des. 92, 491–499 (2014)
Jabbari, M., Khosravi, N., Feizabadi, M., Ajloo, D.: Solubility temperature and solvent dependence and preferential solvation of citrus flavonoid naringin in aqueous DMSO mixtures: an experimental and molecular dynamics simulation study. RSC Adv. 7, 14776–14789 (2017)
Rodríguez, G.A., Delgado, D.R., Martinez, F.: Preferential solvation of indo methacin and naproxen in ethyl acetate + ethanol mixtures according to the IKBI method. Phys. Chem. Liq. 52, 533–545 (2014)
Li, X., Ma, M., Du, C., Zhao, H.: Solubility of cetilistat in neat solvents and preferential solvation in (acetone, isopropanol or acetonitrile) + water co-solvent mixtures. J. Mol. Liq. 242, 618–624 (2017)
Li, X., Chen, J., Chen, G., Zhao, H.: Solubility modelling, solution thermodynamics and preferential solvation of hymecromone in binary solvent mixtures of N,N-dimethylformamide + methanol, ethanol or n-propanol. RSC Adv. 7, 46378–46387 (2017)
Li, X., Wen, X., Cong, Y., Zhao, H.: Solubility modelling, solution thermodynamics and preferential solvation for nitroxoline in solvent mixtures of ethyl acetate + (methanol, ethanol, n-propanol and isopropanol). J. Chem. Thermodyn. 113, 11–19 (2017)
Caires, F.J., Lima, L.S., Carvalho, C.T., Giagio, R.J., Ionashiro, M.: Thermal behaviour of malonic acid, sodium malonate and its compounds with some bivalent transition metal ions. Thermochim. Acta 497, 35–40 (2010)
Limwikrant, W., Nagai, A., Hagiwara, Y., Higashi, K., Yamamoto, K., Moribe, K.: Formation mechanism of a new carbamazepine/malonic acid cocrystal polymorph. Int. J. Pharmaceut. 431, 237–240 (2012)
Shevchenko, A., Miroshnyk, I., Pietila, L., Haarala, J., Salmia, J., Sinervo, K., Mirza, S., Veen, B., Kolehmainen, E., Yliruusi, J.: Diversity in itraconazole cocrystals with aliphatic dicarboxylic acids of varying chain length. Cryst. Growth Des. 13, 4877–4884 (2013)
Trask, A.V., Motherwell, W.D.S., Jones, W.: Pharmaceutical cocrystallization: engineering a remedy for caffeine hydration. Cryst. Growth Des. 5, 1013–1021 (2015)
Wang, Y., Yin, Q., Sun, X., Bao, Y., Gong, J., Hou, B., Wang, Y., Zhang, M., Xie, C., Hao, H.: Measurement and correlation of solubility of thiourea in two solvent mixtures from T = (283.15 to 313.15) K. J. Chem. Thermodyn. 94, 110–118 (2016)
Tang, W., Dai, H., Feng, Y., Wu, S., Bao, Y., Wang, J., Gong, J.: Solubility of tridecanedioic acid in pure solvent systems: an experimental and computational study. J. Chem. Thermodyn. 90, 28–38 (2015)
Wang, G., Wang, Y., Zhang, J., Luan, Q., Ma, Y., Hao, H.: Modeling and simulation of thermodynamic properties of L-alanyl-l-glutamine in different solvents. Ind. Eng. Chem. Res. 53, 3385–3392 (2014)
Apelblat, A., Manzurola, E.: Solubilities of L-aspartic, DL-aspartic, DL-glutamic, p-hydroxybenzoic, o-anisic, p-anisic, and itaconic acids in water from T = 278 K to T = 345 K. J. Chem. Thermodyn. 29, 1527–1533 (1997)
Buchowski, H., Ksiazczak, A., Pietrzyk, S.: Solvent activity along a saturation line and solubility of hydrogen-bonding solids. J. Phys. Chem. 84, 975–979 (1980)
Renon, H., Prausnitz, J.M.: Local compositions in thermodynamic excess functions for liquid mixtures. AIChE J. 14, 135–144 (1968)
Sun, Z., Hao, H., Xie, C., Xu, Z., Yin, Q., Bao, Y., Hou, B., Wang, Y.: Thermodynamic properties of form A and Form B of florfenicol. Ind. Eng. Chem. Res. 53, 13506–13512 (2014)
Barzegar-Jalali, M., Jouyban-Gharamaleki, A.: A general model from theoretical cosolvency models. Int. J. Pharm. 152, 247–250 (1997)
Jouyban-Gharamaleki, A., Acree, W.: Comparison of models for describing multiple peaks in solubility profiles. Int. J. Pharm. 167, 177–182 (1998)
Marcus, Y.: Solvent Mixtures: Properties and Selective Solvation. Marcel Dekker, New York (2002)
Marcus, Y.: On the preferential solvation of drugs and PAHs in binary solvent mixtures. J. Mol. Liq. 140, 61–67 (2008)
Newman, K.E.: Kirkwood–Buff solution theory: derivation and applications. Chem. Soc. Rev. 23, 31–40 (1994)
Ben-Naim, A.: Theory of preferential solvation of nonelectrolytes. Cell Biophys. 12, 255–269 (1988)
Marcus, Y.: Preferential solvation of ibuprofen and naproxen in aqueous 1,2-propanediol. Acta Chim. Slov. 56, 40–44 (2009)
Martínez, A., Jouyban, F.: Preferential solvation of nifedipine in some aqueous co-solvent mixtures. Phys. Chem. Liq. 54, 563–573 (2016)
Marrero, J., Gani, R.: Group-contribution based estimation of pure component properties. Fluid Phase Equilibr. 183–184, 183–208 (2001)
Marcus, Y.: The properties of organic liquids that are relevant to their use as solvating solvents. Chem. Soc. Rev. 22, 409–416 (1993)
Zhu, P., Chen, Y., Zhang, M., Bao, Y., Xie, C., Hou, B., Gong, J., Chen, W.: Measurement and correlation of solubility and solution thermodynamics of 1,3-dimethylurea in different solvents from T = (288.15 to 328.15) K. J. Chem. Thermodyn. 97, 9–16 (2016)
Ouyang, J., Wang, J., Hao, H., Huang, X., Gao, Y., Bao, Y., Wang, Y., Yin, Q.: Determination and correlation of solubility and solution thermodynamics of valnemulin hydrogen tartrate in different pure solvents. Fluid Phase Equilib. 372, 7–14 (2014)
Zhou, Y., Hao, H., Yang, J., Zhu, P., Wang, T., Hou, B., Chuang, X., Wang, J.: Solid–liquid phase equilibrium and mixing properties of 2-cyano-40-methylbiphenyl in pure solvents. J. Chem. Thermodyn. 103, 134–141 (2016)
Nagata, I., Yamada, T., Nakagawa, S.: Excess Gibbs free energies and heats of mixing for binary systems ethyl acetate with methanol, ethanol, 1-propanol, and 2-propanol. J. Chem. Eng. Data 20, 271–275 (1975)
Marcus, Y.: The Properties of Solvents. Wiley, Chichester (2008)
Nikam, P.S., Mahale, T.R., Hasan, M.: Density and viscosity of binary mixtures of ethyl acetate with methanol, ethanol, propan-1-ol, propan-2-ol, butan-1-ol, 2-methylpropan-1-ol, and 2-methylpropan-2-ol at (298.15, 303.15, and 308.15) K. J. Chem. Eng. Data 41, 1055–1058 (1996)
Kamlet, M.J., Taft, R.W.: The solvatochromic comparison method. I. The beta-scale of solvent hydrogen-bond acceptor (HBA) basicities. J. Am. Chem. Soc. 98, 377–383 (1976)
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (21706284), Shandong Scientific Foundation Project (Grant Number ZR2017BB085), Qingdao Postdoctoral Project (Grant Number 2016223) and National Science and Technology Major Project (2016ZX05053-008).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, Z., He, L. & Yu, X. Solubility Measurements and the Dissolution Behavior of Malonic Acid in Binary Solvent Mixtures of (2-Propanol + Ethyl Acetate) by IKBI Calculations. J Solution Chem 48, 427–444 (2019). https://doi.org/10.1007/s10953-019-00853-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-019-00853-7