Abstract
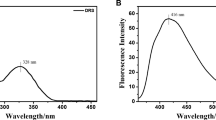
4-[(2-Hydroxy-naphthalen-1-ylmethylene)-amino]-1,5-dimethyl-2-phenyl-1,2-dihydro-pyrazol-3-one (HDDP) was synthesized by the reaction of 4-amino-1,5-dimethyl-2-phenyl-1,2-dihydro-3H-pyrazol-3-one with 2-hydroxy-l-naphthaldehyde. The structure was confirmed by the IR, 1H-NMR, 13C-NMR, and EI-MS spectra and elemental analysis. Physicochemical parameters of the HDDP such as extinction coefficient, oscillator strength, transition dipole moment, Stokes shift, and fluorescence quantum yield in different solvents were studied on the basis of polarities. The interactions of various metal ions with HDDP were also studied using steady state fluorescence measurements. The emission profile reveals that it acts as off–on type fluorescent chemosensor for selective and sensitive detection of Al3+ ions. Complexation stoichiometry and mechanism of enhancement were determined from a Benesi–Hildebrand plot.












Similar content being viewed by others
References
Havrylyuk, D., Roman, O., Lesyk, R.: Synthetic approaches, structure activity relationship and biological applications for pharmacologically attractive pyrazole/pyrazoline–thiazolidine-based hybrids. Eur. J. Med. Chem. 113, 145–166 (2016)
Asiri, A.M., Khan, S.A.: Synthesis, characterization, and in vitro antibacterial activities of macromolecules derived from bis-chalcone. J. Heterocycl. Chem. 49, 1434–1438 (2012)
Nayak, N., Ramprasad, J., Dalimba, U., Yogeeswari, P., Sriram, D.: Synthesis and antimycobacterial screening of new N-(4-(5-aryl-3-(5-methyl-1,3,4-oxadiazol-2-yl)-1H-pyrazol-1-yl)phenyl)-4-amide derivatives. Chin. Chem. Lett. 27, 365–369 (2016)
Kumar, V., Kaur, K.: Fluorinated isoxazolines and isoxazoles: a synthetic perspective. J. Fluorine Chem. 180, 55–97 (2015)
Obasi, L.N., Kaior, G.U., Rhyman, L., Alswaidan, I.A., Ramasami, P.: Synthesis, characterization, antimicrobial screening and computational studies of 4-[3-(4-methoxy-phenyl)-allylideneamino]-1,5-dimethyl-2-phenyl-1,2-dihydro-pyrazol-3-one. J. Mol. Struct. 1120, 180–186 (2016)
Steger, S., Matuszczak, B.: Synthesis of substituted 3-(3-chloro-1H-pyrazol-5-yl)quinoxalin-2(1H)ones. Tetrahedron 706, 763–6768 (2014)
Sztanke, K., Maziarka, A., Osink, A., Sztanke, M.: An insight into synthetic Schiff bases revealing antiproliferative activities in vitro. Bioorg. Med. Chem. 21, 3648–3666 (2013)
Tan, X.J., Hao, X.Q., Zhao, Q.Z., Cheng, S.S., Song, L.Z.: Mono-Schiff-base or di-Schiff-base? Synthesis, spectroscopic, X-ray structural and DFT study of a series of Schiff-bases derived from benzil dihydrazone. J. Mol. Struct. 1099, 373–387 (2015)
Khan, S.A., Asiri, A.M.: Physicochemical properties of novel methyl 2-{(E)-[(2-hydroxynaphthalen-1-yl)methylidene]amino}-4,5,6,7-tetrahydro-1-enzothiophene-3-carboxylate as turn-off fluorometric chemosensor for detection Fe3+ ion. J. Mol. Liq. 243, 85–90 (2017)
Satam, M.A., Telore, R.D., Sekar, N.: Photophysical properties of Schiff’s bases from 3-(1,3-benzothiazol-2-yl)-2-hydroxynaphthalene-1-carbaldehyde. Spectrochim. Acta A 132, 678–686 (2014)
Jia, J.H., Tao, X.M., Li, Y.J., Sheng, W.J., Zheng, Y.F.: Synthesis and third-order optical nonlinearities of ferrocenyl Schiff base. Chem. Phys. Lett. 514, 114–118 (2011)
Cebeci, C., Kilicarslan, F.A., Gurbuz, O., Fırat, Y., Erden, I.: Synthesis and photovoltaic properties of organic photosensitizer using D-π-D type 4,5-diazafluorene ligand and derivatives for efficient dye-sensitized solar cell. Dyes Pigm. 134, 77–82 (2016)
Sharbati, M.T., Rad, M.N.S., Behrouz, S., Gharavi, A., Emami, F.: Near infrared organic light-emitting diodes based on acceptor–donor–acceptor (ADA) using novel conjugated isatin Schiff bases. J. Lumin. 131, 553–558 (2011)
Roy, N., Paul, P.C., Singh, T.S.: Fluorescence characteristics of Schiff base N,N-bis(salicylidene)trans1,2-diaminocyclohexane in the presence of bile acid host. J. Mol. Liq. 21, 1052–1059 (2015)
Keshk, S.M.A.S., Ramadan, A.M., Bondock, S.: Physicochemical characterization of novel Schiff bases derived from developed bacterial cellulose 2,3-dialdehyde. Carbohydr. Polym. 127, 246–251 (2015)
Kupeli, S.: Trace and rare-earth element behaviors during alteration and mineralization in the Attepe iron deposits (Feke-Adana, southern Turkey). J. Geochem. Explor. 105, 51–74 (2010)
Fatemi, S.J.A., Kadir, F.H.A., Williamson, D.J., Moore, G.R.: The uptake, storage, and mobilization of iron and aluminum in biology. Adv. Inorg. Chem. 36, 409–448 (1991)
Stah, T., Brunn, H.: Aluminium content of selected foods and food products. Environ. Sci. Eur. 2, 37–45 (2011)
Ul Hassan, A., Hassan, G., Rasool, Z.: Role of stem cells in treatment of neurological disorder. Int. J. Health Sci. (Qassim) 3, 227–233 (2009)
Walton, J.R.: An aluminum-based rat model for Alzheimer’s disease exhibits oxidative damage, inhibition of PP2A activity, hyperphosphorylated tau, and granulovacuolar degeneration. J. Inorg. Biochem. 101, 1275–1284 (2007)
Ren, J., Tian, H.: Thermally stable merocyanine form of photochromic spiropyran with aluminum ion as a reversible photodriven sensor in aqueous solution. Sensors 7, 3166–3178 (2007)
Bencini, A., Lippolis, V.: Probing biologically and environmentally important metal ions with fluorescent chemosensors: thermodynamic versus optical response selectivity in some study cases. Coord. Chem. Rev. 256, 149–169 (2012)
Sarkar, D., Ghosh, P., Gharami, S., Mondal, T.K., Murmu, N.: A novel coumarin based molecular switch for the sequential detection of Al3+ and F−: application in lung cancer live cell imaging and construction of logic gate. Sens. Actuators, B 242, 338–346 (2017)
Liu, Y., Yang, E.B., Han, R., Zhang, D., Zhao, Y.F.: A new rhodamine-based fluorescent chemosensor for mercury in aqueous media. Chin. Chem. Lett. 25, 1065–1068 (2014)
Chen, C., Liu, H., Zhang, B., Wang, Y., Jiang, Y.: A simple benzimidazole quinoline-conjugate fluorescent chemosensor for highly selective detection of Ag+. Tetrahedron 72, 3980–3985 (2016)
Zheng, X., Lee, K.H., Liu, H., Park, S.Y., Kim, Y.G.: A bis(pyridine-2-ylmethyl) amine-based selective and sensitive colorimetric and fluorescent chemosensor for Cu2+. Sens. Actuators, B 222, 28–34 (2016)
Asiri, A.M., Khan, S.A.: Synthesis, anti-bacterial activities of some novel Schiff bases derived from amino phenazone. Molecules 15, 6850–6858 (2010)
Khan, S.A.: Green synthesis, spectrofluorometric characterization and antibacterial activity of heterocyclic compound from chalcone on the basis of in vitro and quantum chemistry calculation. J. Fluoresc. 27, 929–937 (2017)
Wu, H., Chen, Y., Rao, C., Fan, D., Liu, C.: A selective fluorescent and colorimetric probe for cyanide based on dual-site controlled intramolecular charge transfer–photoinduced electron transfer fluorescence resonance energy transfer. Tetrahedron Lett. 57, 4969–4973 (2016)
Suppan, P.: Invited review solvatochromic shifts: the influence of the medium on the energy of electronic states. J. Photochem. Photobiol., A 50, 293–330 (1990)
Biradar, D.S., Siddlingeshwar, B., Hanagodimath, S.M.: Estimation of ground and excited state dipole moments of some laser dyes. J. Mol. Struct. 875, 108–112 (2008)
Szczepanik, B., Styrcz, S., Gora, M.: Protolytic dissociation of cyanoanilines in the ground and excited state in water and methanol solutions. Spectrochim. Acta, Part A 71, 403–409 (2008)
Mao, M., Xiao, S., Yi, T., Zou, K.: Synthesis and characterization of novel fluorescent BOPIM dyes with large Stokes shift. J. Fluorine Chem. 132, 612–616 (2011)
Ganguly, P.: Photophysics of some cationic dyes in aqueous micellar dispersions of surfactants and in different solvents. J. Mol. Liq. 151, 67–73 (2010)
Siddlingeshwar, B., Hanagodimath, S.M.: Estimation of first excited singlet-state dipole moments of aminoanthraquinones by solvatochromic method. Spectrochim. Acta A 72, 490–495 (2009)
Climent, C., Carreras, A., Alemany, P., Casanova, D.: A push-pull organic dye with a quinoidal thiophene linker: photophysical properties and solvent effects. Chem. Phys. Lett. 663, 45–50 (2016)
Khan, S.A., Asiri, A.M.: Fluorescence quenching of environmentally benign highly fluorescence donor (D)-π-acceptor (A)-π-donor (D) quinoline dye by silver nanoparticles and anionic surfactant in liquid stage. J. Mol. Liq. 221, 381–385 (2016)
Reichardt, C.: Solvatochromic dyes as solvent polarity indicators. Chem. Rev. 94, 2319–2358 (1994)
Zhou, D., Sun, C., Chen, C., Cui, X., Li, W.: Research of a highly selective fluorescent chemosensor for aluminum(III) ions based on photoinduced electron transfer. J. Mol. Struct. 1079, 315–320 (2015)
Choi, Y.W., Lee, J.J., Nam, E., Lim, M.H., Kim, C.: A fluorescent chemosensor for Al3+ based on julolidine and tryptophan moieties. Tetrahedron 72, 1998–2005 (2016)
Liao, Z.C., Yang, Z.Y., Li, Y., Wang, B.D., Zhou, Q.X.: A simple structure fluorescent chemosensor for high selectivity and sensitivity of aluminum ions. Dyes Pigm. 97, 124–128 (2013)
Marwani, H.M., Asiri, A.M., Khan, S.A.: Spectral, stoichiometric ratio, physicochemical, polarity and photostability studies of newly synthesized chalcone dye in organized media. J. Lumin. 136, 296–302 (2013)
Acknowledgements
The authors are thankful to the Chemistry Department at King Abdulaziz University for providing research facilities.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Asiri, A.M., Sobahi, T.R., Al-Amari, M.M. et al. Physicochemical Investigation of HDDP Azomethine Dye as Turn-On Fluorescent Chemosensor for High Selectivity and Sensitivity of Al3+ Ions. J Solution Chem 47, 1711–1724 (2018). https://doi.org/10.1007/s10953-018-0805-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-018-0805-1