Abstract
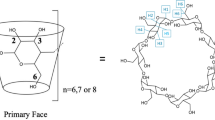
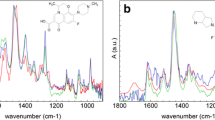
Researchers developing software to predict the binding constants of small molecules for proteins have, in recent years, turned to host–guest systems as simple, computationally tractable model systems to test and improve these computational methods. However, taking full advantage of this strategy requires aqueous host–guest systems that probe a greater diversity of chemical interactions. Here, we advance the development of an experimental platform to generate such systems by building on the cyclodextrin (CD) class of hosts. The secondary face derivative mono-3-carboxypropionamido-β-cyclodextrin (CP-β-CD) was synthesized in a one-pot strategy with 87% yield, and proved to have much greater aqueous solubility than native β-CD. The complexation of anionic CP-β-CD with the cationic drug rimantadine hydrochloride was explored using one- and two-dimensional nuclear magnetic resonance; NOESY analysis showed secondary face binding of the ammonium moiety of the guest, based on cross-correlations between the amic acid functionality and the side-chain of rimantadine. Isothermal titration calorimetry was furthermore used to determine the standard Gibbs energy and enthalpy for this binding reaction, and the results were compared with those of rimantadine with native β-CD.








Similar content being viewed by others
References
Gilson, M.K., Zhou, H.-X.: Calculation of protein–ligand binding affinities. Ann. Rev. Biophys. Biomol. Struct. 36, 21–42 (2007)
Kuntz, I.D., Blaney, J.M., Oatley, S.J., Langridge, R., Ferrin, T.E.: A geometric approach to macromolecule–ligand interactions. J. Mol. Biol. 161, 269–288 (1982)
Goodsell, D.S., Olson, A.J.: Automated docking of substrates to proteins by simulated annealing. Protein Struct. Funct. Gen. 8, 195–202 (1990)
Abagyan, R., Totrov, M., Kuznetsov, D.: ICM—a new method for protein modeling and design. Applications to docking and structure prediction from the distorted native conformation. J. Comput. Chem. 15, 488–506 (1994)
Apostolakis, J., Pluckthun, A., Caflisch, A.: Docking small ligands in flexible binding sites. J. Comput. Chem. 19, 21–37 (1998)
Charifson, P.S., Corkery, J.J., Murcko, M.A., Walters, W.P.: Consensus scoring: a method for obtaining improved hit rates from docking databases of three-gimensional structures into proteins. J. Med. Chem. 42, 5100–5109 (1999)
Clark, K.P.: Ajay: flexible ligand docking without parameter adjustment across four ligand–receptor complexes. J. Comput. Chem. 16, 1210–1226 (1995)
David, L., Luo, R., Gilson, M.K.: Ligand–receptor docking with the Mining Minima optimizer. J. Comput. Aided Mol. Des. 15, 157–171 (2001)
Trott, O., Olson, A.J.: AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 31, 455–461 (2010). https://doi.org/10.1002/jcc.21334
Jain, A.N.: Surflex: fully automatic flexible molecular docking using a molecular similarity-based search engine. J. Med. Chem. 46, 499–511 (2003). https://doi.org/10.1021/jm020406h
Halgren, T.A., Murphy, R.B., Friesner, R.A., Beard, H.S., Frye, L.L., Pollard, W.T., Banks, J.L.: Glide: a new approach for rapid, accurate docking and scoring. 2. Enrichment factors in database screening. J. Med. Chem. 47, 1750–1759 (2004). https://doi.org/10.1021/jm030644s
Abel, R., Wang, L., Mobley, D.L., Friesner, R.A.: A critical review of validation, blind testing, and real-world use of Alchemical protein–ligand binding free energy calculations. Curr. Top. Med. Chem. 17, 2577–2585 (2017)
Gallicchio, E., Lapelosa, M., Levy, R.M.: Binding energy distribution analysis method (BEDAM) for estimation of protein–ligand binding affinities. J. Chem. Theory Comput. 6, 2961–2977 (2010). https://doi.org/10.1021/ct1002913
Gilson, M.K., Given, J.A., Bush, B.L., McCammon, J.A.: The statistical-thermodynamic basis for computation of binding affinities: a critical review. Biophys. J. 72, 1047–1069 (1997)
Hansen, N., van Gunsteren, W.F.: Practical aspects of free-energy calculations: a review. J. Chem. Theory Comput. 10, 2632–2647 (2014). https://doi.org/10.1021/ct500161f
Jorgensen, W.L., Buckner, J.K., Boudon, S., Tirado-Rives, J.: Efficient computation of absolute free energies of binding by computer simulations. Application to the methane dimer in water. J. Chem. Phys. 89, 3742–3746 (1988). https://doi.org/10.1063/1.454895
Lau, F.T.K., Karplus, M.: Molecular recognition in proteins: simulation analysis of substrate binding by a tyrosyl-tRNA synthetase mutant. J. Mol. Biol. 236, 1049–1066 (1994). https://doi.org/10.1016/0022-2836(94)90011-6
Simonson, T., Archontis, G., Karplus, M.: Free energy simulations come of age: protein–ligand recognition. Acc. Chem. Res. 35, 430–437 (2002). https://doi.org/10.1021/ar010030m
Tembe, B.L., McCammon, J.A.: Ligand–receptor interactions. Comput. Chem. 8, 281–283 (1984)
Woo, H.-J., Roux, B.: Calculation of absolute protein–ligand binding free energy from computer simulations. Proc. Natl. Acad. Sci. USA 102, 6825–6830 (2005). https://doi.org/10.1073/pnas.0409005102
Homeyer, N., Stoll, F., Hillisch, A., Gohlke, H.: Binding free energy calculations for lead optimization: assessment of their accuracy in an industrial drug design context. J. Chem. Theory Comput. 10, 3331–3344 (2014). https://doi.org/10.1021/ct5000296
Kuhn, B., Tichý, M., Wang, L., Robinson, S., Martin, R.E., Kuglstatter, A., Benz, J., Giroud, M., Schirmeister, T., Abel, R., Diederich, F., Hert, J.: Prospective evaluation of free energy calculations for the prioritization of cathepsin L inhibitors. J. Med. Chem. 60, 2485–2497 (2017). https://doi.org/10.1021/acs.jmedchem.6b01881
Williams-Noonan, B.J., Yuriev, E., Chalmers, D.K.: Free energy methods in drug design: prospects of “Alchemical Perturbation” in medicinal chemistry. J. Med. Chem. (2017). https://doi.org/10.1021/acs.jmedchem.7b00681
Cournia, Z., Allen, B., Sherman, W.: Relative binding free energy calculations in drug discovery: recent advances and practical considerations. J. Chem. Inf. Model. 57, 2911–2937 (2017). https://doi.org/10.1021/acs.jcim.7b00564
Sherborne, B., Shanmugasundaram, V., Cheng, A.C., Christ, C.D., DesJarlais, R.L., Duca, J.S., Lewis, R.A., Loughney, D.A., Manas, E.S., McGaughey, G.B., Peishoff, C.E., van Vlijmen, H.: Collaborating to improve the use of free-energy and other quantitative methods in drug discovery. J. Comput. Aided Mol. Des. 30, 1139–1141 (2016). https://doi.org/10.1007/s10822-016-9996-y
Lybrand, T.P., McCammon, J.A., Wipff, G.: Theoretical calculation of relative binding affinity in host–guest systems. Proc. Natl. Acad. Sci. USA 83, 833–835 (1986)
Pitera, J., Kollman, P.: Designing an optimum guest for a host using multimolecule free energy calculations: predicting the best ligand for Rebek’s “tennis ball”. J. Am. Chem. Soc. 120, 7557–7567 (1998). https://doi.org/10.1021/ja973028s
Kaminski, G.A., Jorgensen, W.L.: Host–guest chemistry of rotaxanes and catenanes: application of a polarizable all-atom force field to cyclobis(paraquat-p-phenylene) complexes with disubstituted benzenes and biphenyls. J. Chem. Soc. Perkin Trans. 2, 2365–2375 (1999). https://doi.org/10.1039/A905160K
Chen, W., Chang, C.E., Gilson, M.K.: Calculation of cyclodextrin binding affinities: energy, entropy, and implications for drug design. Biophys. J. 87, 3035–3049 (2004). https://doi.org/10.1529/biophysj.104.049494
Chang, C.-E., Gilson, M.K.: Free energy, entropy, and induced fit in host-guest recognition: calculations with the second-generation mining minima algorithm. J. Am. Chem. Soc. 126, 13156–13164 (2004)
Rogers, K.E., Ortiz-Sánchez, J.M., Baron, R., Fajer, M., de Oliveira, C.A.F., McCammon, J.A.: On the role of dewetting transitions in host–guest binding free energy calculations. J. Chem. Theory Comput. 9, 46–53 (2013). https://doi.org/10.1021/ct300515n
Wickstrom, L., He, P., Gallicchio, E., Levy, R.M.: Large scale affinity calculations of cyclodextrin host–guest complexes: understanding the role of reorganization in the molecular recognition process. J. Chem. Theory Comput. 9, 3136–3150 (2013). https://doi.org/10.1021/ct400003r
Bell, D.R., Qi, R., Jing, Z., Xiang, J.Y., Mejias, C., Schnieders, M.J., Ponder, J.W., Ren, P.: Calculating binding free energies of host–guest systems using AMOEBA polarizable force field. Phys. Chem. Chem. Phys. 18, 30261–30269 (2016). https://doi.org/10.1039/c6cp02509a
Henriksen, N.M., Gilson, M.K.: Evaluating force field performance in thermodynamic calculations of cyclodextrin host-guest binding: water models, partial charges, and host force field parameters. J. Chem. Theory Comput. 13, 4253–4269 (2017). https://doi.org/10.1021/acs.jctc.7b00359
Mobley, D.L., Gilson, M.K.: Predicting binding free energies: frontiers and benchmarks. Ann. Rev. Biophys. 46, 531–558 (2017)
Gao, K., Yin, J., Henriksen, N.M., Fenley, A.T., Gilson, M.K.: Binding enthalpy calculations for a neutral host–guest pair yield widely divergent salt effects across water models. J. Chem. Theory Comput. 11, 4555–4564 (2015)
Yin, J., Fenley, A.T., Henriksen, N.M., Gilson, M.K.: Toward improved force-field accuracy through sensitivity analysis of host–guest binding thermodynamics. J. Phys. Chem. B 119, 10145–10155 (2015). https://doi.org/10.1021/acs.jpcb.5b04262
Bradshaw, R.T., Essex, J.W.: Evaluating parametrization protocols for hydration free energy calculations with the AMOEBA polarizable force field. J. Chem. Theory Comput. 12, 3871–3883 (2016). https://doi.org/10.1021/acs.jctc.6b00276
Buck, M., Bouguet-Bonnet, S., Pastor, R.W., MacKerell, A.D.: Importance of the CMAP correction to the CHARMM22 protein force field: dynamics of hen lysozyme. Biophys. J. 90, L36–L38 (2006). https://doi.org/10.1529/biophysj.105.078154
Jorgensen, W.L., Tirado-Rives, J.: The OPLS [optimized potentials for liquid simulations] potential functions for proteins, energy minimizations for crystals of cyclic peptides and crambin. J. Am. Chem. Soc. 110, 1657–1666 (1988). https://doi.org/10.1021/ja00214a001
Kang, M., Smith, P.E.: A Kirkwood-Buff derived force field for amides. J. Comput. Chem. 27, 1477–1485 (2006). https://doi.org/10.1002/jcc.20441
Oostenbrink, C., Villa, A., Mark, A.E., van Gunsteren, W.F.: A biomolecular force field based on the free enthalpy of hydration and solvation: the GROMOS force-field parameter sets 53A5 and 53A6. J. Comput. Chem. 25, 1656–1676 (2004). https://doi.org/10.1002/jcc.20090
Piana, S., Donchev, A.G., Robustelli, P., Shaw, D.E.: Water dispersion interactions strongly influence simulated structural properties of disordered protein States. J. Phys. Chem. B 119, 5113–5123 (2015). https://doi.org/10.1021/jp508971m
Ploetz, E.A., Smith, P.E.: A Kirkwood-Buff force field for the aromatic amino acids. Phys. Chem. Chem. Phys. PCCP 13, 18154–18167 (2011). https://doi.org/10.1039/c1cp21883b
Song, D., Wang, W., Ye, W., Ji, D., Luo, R., Chen, H.-F.: ff14IDPs force field improving the conformation sampling of intrinsically disordered proteins. Chem. Biol. Drug Des. 89, 5–15 (2017). https://doi.org/10.1111/cbdd.12832
Weerasinghe, S., Gee, M., Kang, M., Bentenitis, N., Smith, P.: Developing Force Fields from the Microscopic Structure of Solutions: The Kirkwood-Buff Approach. Modelling Solvent Environment, pp. 55–76. Wiley-VCH, Weinheim (2010). https://doi.org/10.1002/9783527629251.ch3
Davis, M.E., Brewster, M.E.: Cyclodextrin-based pharmaceutics: past, present and future. Nat. Rev. Drug Discov. 3, 1023–1035 (2004). https://doi.org/10.1038/nrd1576
Brewster, M.E., Loftsson, T.: Cyclodextrins as pharmaceutical solubilizers. Adv. Drug Deliv. Rev. 59, 645–666 (2007). https://doi.org/10.1016/j.addr.2007.05.012
Loftsson, T., Brewster, M.E.: Pharmaceutical applications of cyclodextrins: basic science and product development. J. Pharm. Pharmacol. 62, 1607–1621 (2010). https://doi.org/10.1111/j.2042-7158.2010.01030.x
Yanli, C., Caixia, W., Jianwei, M., Yongping, Y.: A facile and practical approach to randomly methylated beta-cyclodextrin. J. Chem. Technol. Biotechnol. 85, 248–251 (2010). https://doi.org/10.1002/jctb.2295
Khan, A.R., Forgo, P., Stine, K.J., D’Souza, V.T.: Methods for selective modifications of cyclodextrins. Chem. Rev. 98, 1977–1996 (1998). https://doi.org/10.1021/cr970012b
Ashton, P.R., Königer, R., Stoddart, J.F., Alker, D., Harding, V.D.: Amino acid derivatives of β-cyclodextrin. J. Org. Chem. 61, 903–908 (1996). https://doi.org/10.1021/jo951396d
Carrazana, J., Jover, A., Meijide, F., Soto, V.H., Tato, J.V.: Complexation of adamantyl compounds by β-cyclodextrin and monoaminoderivatives. J. Phys. Chem. B 109, 9719–9726 (2005). https://doi.org/10.1021/jp0505781
Kantonen, S.A., Henriksen, N.M., Gilson, M.K.: Accounting for apparent deviations between calorimetric and van’t Hoff enthalpies. Biochim. Biophys. Acta (2017). https://doi.org/10.1016/j.bbagen.2017.11.020
Dodzuik, E.: Cyclodextrins and Their Complexes. Wiley-VCH, Weinheim (2006)
Hwang, T.L., Shaka, A.J.: Water suppression that works. Excitation sculpting using arbitrary wave-forms and pulsed-field gradients. J. Magn. Reson. A 112, 275–279 (1995). https://doi.org/10.1006/jmra.1995.1047
Wiseman, T., Williston, S., Brandts, J.F., Lin, L.N.: Rapid measurement of binding constants and heats of binding using a new titration calorimeter. Anal. Biochem. 179, 131–137 (1989). https://doi.org/10.1016/0003-2697(89)90213-3
Acknowledgements
M.K.G. has an equity interest in, and is a cofounder and scientific advisor of, VeraChem LLC. NMR spectra were collected at the UCSD Skaggs School of Pharmacy and Pharmaceutical Sciences NMR Facility.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kellett, K., Kantonen, S.A., Duggan, B.M. et al. Toward Expanded Diversity of Host–Guest Interactions via Synthesis and Characterization of Cyclodextrin Derivatives. J Solution Chem 47, 1597–1608 (2018). https://doi.org/10.1007/s10953-018-0769-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-018-0769-1