Abstract
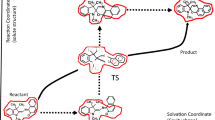
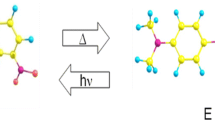
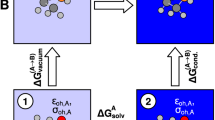
The dynamic solvent effect often arises in solution reactions, where coupling between chemical reaction and solvent fluctuation plays a decisive role in the reaction kinetics. In this study, the Z/E isomerization reaction of nitoroazobenzene and benzylideneanilines in the ground state was computationally studied by molecular dynamics simulations. The non-equilibrium solvation effect was analyzed using two approaches: (1) metadynamics Gibbs energy surface exploration and (2) solvation Gibbs energy evaluation using a frozen solvation droplet model. The solute–solvent coupling parameter (Ccoupled) was estimated by the ratio of the solvent fluctuation Gibbs energy over the corresponding isomerization activation Gibbs energy. The results were discussed in comparison with the ones estimated by means of the analytical models based on a reaction–diffusion equation with a sink term. The second approach using a frozen solvation droplet reached qualitative agreement with the analytical models, while the first metadynamics approach failed. This is because the second approach explicitly considers the non-equilibrium solvation in the droplet, which consists of a solute at the reactant geometry immersed in the pre-organized solvents fitted with the solute at the transition state geometry.





Similar content being viewed by others
References
Calef, D.F., Deutch, J.M.: Diffusion-controlled reactions. Ann. Rev. Phys. Chem. 34, 393–524 (1983)
Orr-Ewing, A.J.: Bimolecular chemical reaction dynamics in liquids. J. Chem. Phys. 140, 090901 (2014)
Weinberg, N.: Theoretical models in high pressure reaction kinetics: from empirical correlations to molecular dynamics. High-Pressure Sci. Technol. (in Japanese) 8(2), 86–95 (1998)
Kramers, H.: Brownian motion in a field of force and the diffusion model of chemical reactions. Physica 7, 284–304 (1940)
Asano, T., Crosstick, K., Furuta, H., Matsuo, K., Sumi, H.: Effects of solvent fluctuations on the rate of thermal Z/E isomerization of azobenzenes and N-benzylideneanilines. Bull. Chem. Soc. Jpn 69, 551–560 (1996)
Asano, T., Furuta, H., Sumi, H.: Two-step mechanism in single-step osomerizations. Kinetics in a highly viscous liquid phase. J. Am. Chem. Soc. 116, 5545–5550 (1994)
Peters, B.: Common features of extraordinary rate theories. J. Phys. Chem. B 119, 6349–6356 (2015)
Hänggi, P., Talkner, P., Borkovec, M.: Reaction-rate theory: fifty years after Kramers. Rev. Mod. Phys. 62, 251–341 (1990)
Berne, B.J., Borkovec, M., Straub, J.E.: Classical and modern methods in reaction rate theory. J. Phys. Chem. 92, 3711–3725 (1988)
Pollak, E., Talkner, P.: Reaction rate theory: what it was, where is it today, and where is it going? Chaos 15, 026116 (2005)
Grote, R.F., Hynes, J.T.: Reactive modes in condensed phase reactions. J. Chem. Phys. 74, 4465–4475 (1981)
Hynes, J.T.: The theory of reactions in solutions. In: Baer, M. (ed.) Theory of Chemical Reaction Dynamics, vol. 4, pp. 171–234. CRC Press, Boca Raton (1985)
van der Zwan, G., Hynes, J.T.: A simple dipole isomerization model for non-equilibrium solvation dynamics in reactions in polar solvents. Chem. Phys. 90, 21–35 (1984)
Hynes, J.T.: Molecules in motion: chemical reaction and allied dynamics in solution and elsewhere. Ann. Rev. Phys. Chem. 66, 1–20 (2015)
Pollak, E.: Theory of activated rate processes: a new derivation of Kramers’ expression. J. Chem. Phys. 85, 865–867 (1986)
Agmon, N., Hopfield, J.J.: Transient kinetics of chemical reactions with bounded diffusion perpendicular to the reaction coordinate: intramolecular processes with slow conformational changes. J. Chem. Phys. 78, 6947–6959 (1983)
Sumi, H., Marcus, R.A.: Dynamical effects in electron transfer reactions. J. Chem. Phys. 84, 4894–4914 (1986)
Nadler, W., Marcus, R.A.: Dynamical effects in electron transfer reactions. II. Numerical solution. J. Chem. Phys. 86, 3906–3924 (1987)
Basilevsky, M.V., Ryaboy, V.M., Weinberg, N.N.: Kinetics of chemical reactions in condensed media in the framework of the two-dimensional stochastic model. J. Phys. Chem. 94, 8734–8740 (1990)
Weidenmüller, H.A., Zhang, J.-S.: Stationary diffusion over a multidimensional potential barrier: a generalization of Kramers’ formula. J. Stat. Phys. 34, 191–201 (1984)
Langer, J.S.: Theory of the condensation point. Ann. Phys. 41, 108–157 (1967)
Berezhkovskii, A., Zitserman, V.Y.: Anomalous regime for decay of the metastable state: an extension of multidimensional Kramer’s theory. Chem. Phys. Lett. 158, 369–374 (1989)
Biswas, R., Bagchi, B.: Activated barrier crossing dynamics in slow, viscous liquids. J. Chem. Phys. 105, 7543–7549 (1996)
Asano, T.: Kinetics in highly viscous solutions: dynamic solvent effects in slow reactions. Pure Appl. Chem. 71, 1691–1704 (1999)
Sumi, H., Asano, T.: General expression for rates of solution reactions influenced by slow solvent fluctuations, and its experimental evidence. Electorochim. Acta 42, 2763–2777 (1997)
Sumi, H., Asano, T.: An experimental examination of Biswas-Bagchi’s prediction on the viscosity dependence of the rate of activated barrier surmounting in viscous liquids. Chem. Phys. Lett. 294, 493–498 (1998)
Chipot, C., Pohorille, A. (eds.): Free Energy Calculations. Springer, New York (2007)
Bolhuis, P.G., Chandler, D., Dellago, C., Geissler, P.L.: Transition path sampling: throwing ropes over rough mountain passes in the dark. Ann. Rev. Phys. Chem. 53, 291–318 (2002)
Hamelberg, D., Mongan, J., McCammon, A.J.: Accelerated molecular dynamics: a promising and efficient simulation method for biomolecules. J. Chem. Phys. 120, 11919–11929 (2004)
Laio, A., Parrinello, M.: Metadynamics: a method to stimulate rare events and reconstruct the free energy in biophysics, chemistry and material science. Proc. Natl. Acad. Sci. USA 99, 12562–12566 (2002)
Bussi, G., Branduardi, D.: Free energy calculations with metadynamics: theory and practice. In: Parrill, A.L., Lipkowitz, K.B. (eds.) Reviews in Computational Chemistry, vol. 28. Wiley, New York (2015)
Barducci, A., Bussi, G., Parrinello, M.: Well-tempered metadynamics: a smoothly converging and tunable free-energy method. Phys. Rev. Lett. 100, 020603 (2008)
Bussi, G., Gervasio, F.L., Laio, A., Parrinello, M.: Free-energy landscape for β hairpin folding from combined parallel tempering and metadynamics. J. Am. Chem. Soc. 128, 13435–13441 (2006)
Piana, S., Laio, A.: A bias-exchange approach to protein folding. J. Phys. Chem. B 111, 4553–4559 (2007)
Laio, A., Rodrigues-Fortea, A., Gervasio, F.L., Ceccarelli, M., Parrinello, M.: Assessing the accuracy of metadynamics. J. Phys. Chem. B 109, 6714–6721 (2005)
Bian, Y., Zhang, J., Wang, J., Wang, W.: On the accuracy of metadynamics and its variations in a protein folding process. Mol. Simul. 41, 752–763 (2015)
Asano, T., Yano, T., Okada, T.: Mechanistic study of thermal Z-E isomerization of azobenzenes by high-pressure kinetics. J. Am. Chem. Soc. 104, 4900–4904 (1982)
Asano, T., Okada, T.: Thermal Z-E isomerization of azobenzenes. The pressure, solvent, and substituent effects. J. Org. Chem. 49, 4387–4391 (1984)
Asano, T., Okada, T., Herkstroeter, W.G.: Mechanism of geometrical isomerization about the carbon-nitrogen double bond. J. Org. Chem. 54, 379–383 (1989)
Asano, T., Furuta, H., Hofmann, H.-J., Cimiraglia, R., Tsuno, Y., Fujio, M.: Mechanism of thermal Z/E isomerization of substituted N-benzylideneanilines. Nature of the activated complex with an sp-hybridized nitrogen atom. J. Org. Chem. 58, 4418–4423 (1993)
Asano, T., Matsuo, K., Sumi, H.: Effects of solvent fluctuations on the rate of the thermal Z/E isomerization of N-benzylideneanilines in a highly viscous liquid hydrocarbon. Bull. Chem. Soc. Jpn 70, 239–244 (1997)
Asano, T., Okada, T.: Further kinetic evidence for the competitive rotational and inversional Z-E isomerization of substituted azobenzenes. J. Org. Chem. 51, 4454–4458 (1986)
Yamataka, H., Ammal, S.C., Asano, T., Ohga, Y.: Thermal isomerization at a CN double bond. How does the mechanism vary with the substituents? Bull. Chem. Soc. Jpn 78, 1851–1855 (2005)
Case, D.A., Darden, T.A., Cheatham III, T.E., Simmerling, C.L., Wang, J., Duke, R.E., Luo, R., Walker, R.C., Zhang, W., Merz, K.M., Roberts, B., Hayik, S., Roitberg, A., Seabra, G., Swails, J., Goetz, A.W., Kolossváry, I., Wong, K.F., Paesani, F., Vanicek, J., Wolf, R.M., Liu, J., Wu, X., Brozell, S.R., Steinbrecher, T., Gohlke, H., Cai, Q., Ye, X., Wang, J., Hsieh, M.-J., Cui, G., Roe, D.R., Mathews, D.H., Seetin, M.G., Salomon-Ferrer, R., Sagui, C., Babin, V., Luchko, T., Gusarov, S., Kovalenko, A., Kollman, P.A.: AMBER 12. University of California, San Francisco (2012)
Wang, J., Wolf, R.M., Caldwell, J.W., Kollman, P.A., Case, D.A.: Development and testing of a general AMBER force field. J. Comp. Chem. 25, 1157–1174 (2004)
Wang, J., Wang, W., Kollman, P.A., Case, D.A.: Automatic atom type and bond type perception in molecular mechanical calculations. J. Mol. Graph. Model. 25, 247–260 (2006)
Frisch, M.J., Trucks, G.W., Schlegel, H.B., Scuseria, G.E., Robb, M.A., Cheeseman, J.R., Scalmani, G., Barone, V., Mennucci, B., Petersson, G.A., Nakatsuji, H., Caricato, M., Li, X., Hratchian, H.P., Izmaylov, A.F., Bloino, J., Zheng, G., Sonnenberg, J.L., Hada, M., Ehara, M., Toyota, K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Honda, Y., Kitao, O., Nakai, H., Vreven, T., Montgomery, Jr., J.A., Peralta, J.E., Ogliaro, F., Bearpark, J., Heyd, J., Brothers, E., Kudin, K.N., Staroverov, V.N., Kobayashi, R., Normand, J., Raghavachari, K., Rendell, A., Burant, J.C., Iyengar, S.S., Tomasi, J., Cossi, M., Rega, N., Millam, J.M., Klene, M., Knox, J.E., Cross, J.B., Bakken, V., Adamo, C., Jaramillo, J., Gomperts, R., Stratmann, R.E., Yazyev, O., Austin, A.J., Cammi, R., Pomelli, C., Ochterski, J.W., Martin, R.L., Morokuma, K., Zakrzewski, V.G., Voth, G.A., Salvador, P., Ortiz, J.V., Cioslowski, J., Fox, D.J.: Gaussian 09 Revision B.1. Gaussian Inc., Wallingford CT (2009)
Tribello, G.A., Bonomi, M., Branduardi, D., Camilloni, C., Bussi, G.: PLUMED2: new feathers for an old bird. Comp. Phys. Comm. 185, 604–613 (2014)
Ohue, M., Shimoda, T., Suzuki, S., Matsuzaki, Y., Ishida, T., Akiyama, Y.: MEGADOCK 4.0: an ultra-high-performance protein–protein docking software for heterogeneous supercomputers. Bioinformatics 30, 3281–3283 (2014)
Matubayasi, N., Nakahara, M.: Theory of solutions in the energetic representation. I. Formulation. J. Chem. Phys. 113, 6070–6081 (2000)
Dhaliwal, M., Basilevsky, M.V., Weinberg, N.: Dynamics effects of nonequilibrium solvation: potential and free energy surfaces for Z/E isomerization in solvent–solute coordinates. J. Chem. Phys. 126, 234505 (2007)
Sumi, H.: Theory on reaction rates in nonthermalized steady states during conformational fluctuations in viscous solvents. J. Phys. Chem. 95, 3334–3350 (1991)
Acknowledgements
The authors thank to Prof. Mikhail Basilevsky (Russian Academy of Sciences) for his generous permission to use his program for evaluation of the parameter BW-C2. Helpful discussions with Professor Emeritus Tsutomu Asano (Oita University, Japan) and Prof. Noam Weinberg (University College of Fraser Valley, Canada) are greatly acknowledged. One of the authors (Y.S.) was financially supported by Grant-in-Aids for Scientific Research (C) (15K05434) from the Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shigemitsu, Y., Ohga, Y. Computational Analysis of Solute–Solvent Coupling Magnitude in the Z/E Isomerization Reaction of Nitroazobenzene and Benzylideneanilines. J Solution Chem 47, 127–139 (2018). https://doi.org/10.1007/s10953-018-0711-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-018-0711-6